| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prostaglandin G/H synthase 1 |
|---|
| Ligand | BDBM50022271 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1542270 (CHEMBL3745492) |
|---|
| IC50 | 67±n/a nM |
|---|
| Citation |  Zhang, Y; Tortorella, MD; Liao, J; Qin, X; Chen, T; Luo, J; Guan, J; Talley, JJ; Tu, Z Synthesis and Evaluation of Novel Erlotinib-NSAID Conjugates as More Comprehensive Anticancer Agents. ACS Med Chem Lett6:1086-90 (2015) [PubMed] Article Zhang, Y; Tortorella, MD; Liao, J; Qin, X; Chen, T; Luo, J; Guan, J; Talley, JJ; Tu, Z Synthesis and Evaluation of Novel Erlotinib-NSAID Conjugates as More Comprehensive Anticancer Agents. ACS Med Chem Lett6:1086-90 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prostaglandin G/H synthase 1 |
|---|
| Name: | Prostaglandin G/H synthase 1 |
|---|
| Synonyms: | COX1 | Cyclooxygenase-1 | Cyclooxygenase-1 (COX-1) | PGH1_SHEEP | PTGS1 | Prostaglandin G/H synthase (cyclooxygenase) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 68868.60 |
|---|
| Organism: | Ovis aries (Sheep) |
|---|
| Description: | n/a |
|---|
| Residue: | 600 |
|---|
| Sequence: | MSRQSISLRFPLLLLLLSPSPVFSADPGAPAPVNPCCYYPCQHQGICVRFGLDRYQCDCT
RTGYSGPNCTIPEIWTWLRTTLRPSPSFIHFLLTHGRWLWDFVNATFIRDTLMRLVLTVR
SNLIPSPPTYNIAHDYISWESFSNVSYYTRILPSVPRDCPTPMDTKGKKQLPDAEFLSRR
FLLRRKFIPDPQSTNLMFAFFAQHFTHQFFKTSGKMGPGFTKALGHGVDLGHIYGDNLER
QYQLRLFKDGKLKYQMLNGEVYPPSVEEAPVLMHYPRGIPPQSQMAVGQEVFGLLPGLML
YATIWLREHNRVCDLLKAEHPTWGDEQLFQTARLILIGETIKIVIEEYVQQLSGYFLQLK
FDPELLFGAQFQYRNRIAMEFNQLYHWHPLMPDSFRVGPQDYSYEQFLFNTSMLVDYGVE
ALVDAFSRQPAGRIGGGRNIDHHILHVAVDVIKESRVLRLQPFNEYRKRFGMKPYTSFQE
LTGEKEMAAELEELYGDIDALEFYPGLLLEKCHPNSIFGESMIEMGAPFSLKGLLGNPIC
SPEYWKASTFGGEVGFNLVKTATLKKLVCLNTKTCPYVSFHVPDPRQEDRPGVERPPTEL
|
|
|
|---|
| BDBM50022271 |
|---|
| n/a |
|---|
| Name | BDBM50022271 |
|---|
| Synonyms: | 2-(3-Benzoylphenyl)propionic acid | 2-(3-benzoylphenyl)propanoic acid | 3-Benzoyl-alpha-methylbenzeneacetic acid | 3-Benzoylhydratropic acid | CHEMBL571 | Dexketoprofen trometamol | KETOPROFEN | L'Acide (benzoyl-3-phenyl)-2-propionique | Orudis (TN) | m-Benzoylhydratropic acid |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H14O3 |
|---|
| Mol. Mass. | 254.2806 |
|---|
| SMILES | CC(C(O)=O)c1cccc(c1)C(=O)c1ccccc1 |
|---|
| Structure | 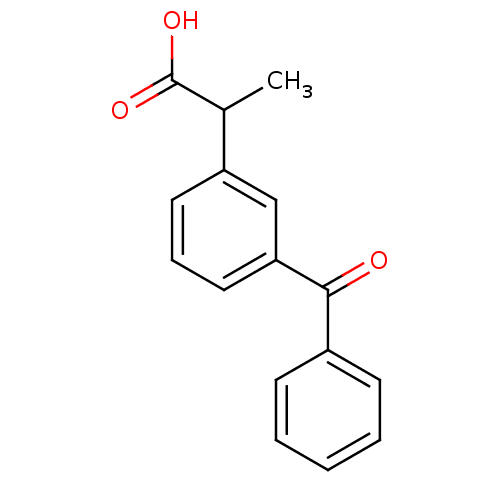
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhang, Y; Tortorella, MD; Liao, J; Qin, X; Chen, T; Luo, J; Guan, J; Talley, JJ; Tu, Z Synthesis and Evaluation of Novel Erlotinib-NSAID Conjugates as More Comprehensive Anticancer Agents. ACS Med Chem Lett6:1086-90 (2015) [PubMed] Article
Zhang, Y; Tortorella, MD; Liao, J; Qin, X; Chen, T; Luo, J; Guan, J; Talley, JJ; Tu, Z Synthesis and Evaluation of Novel Erlotinib-NSAID Conjugates as More Comprehensive Anticancer Agents. ACS Med Chem Lett6:1086-90 (2015) [PubMed] Article