

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126049 (4-[(1S,2R)-1-Hydroxycarbamoyl-2-((1S,2R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126054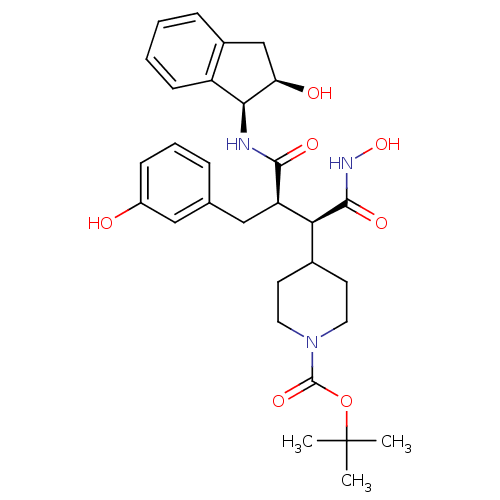 (4-[(R)-1-Hydroxycarbamoyl-2-((3S,3aR)-2-hydroxy-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126047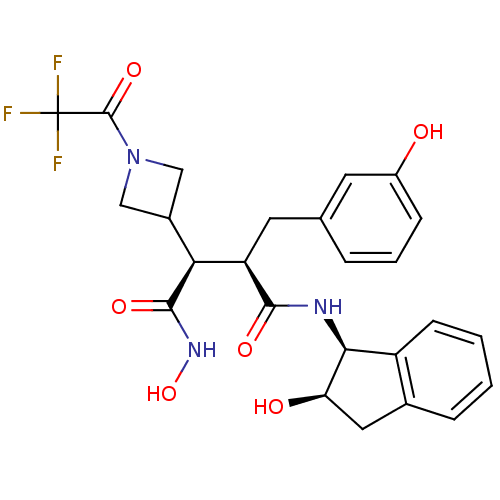 ((2R,3S)-N*4*-Hydroxy-2-(3-hydroxy-benzyl)-N*1*-((1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126053 ((2R,3S)-2-(1-Acetyl-piperidin-4-yl)-N*1*-hydroxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126057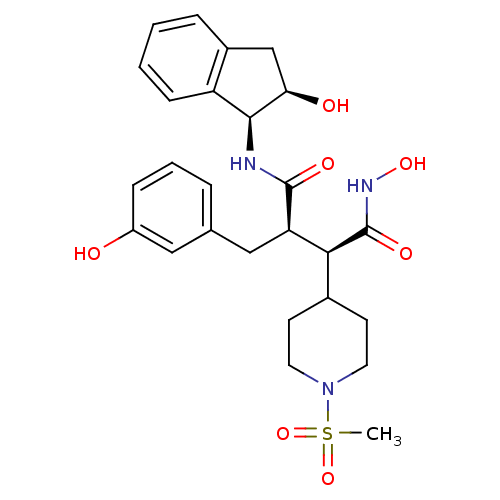 ((2R,3S)-N*4*-Hydroxy-2-(3-hydroxy-benzyl)-N*1*-((1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126055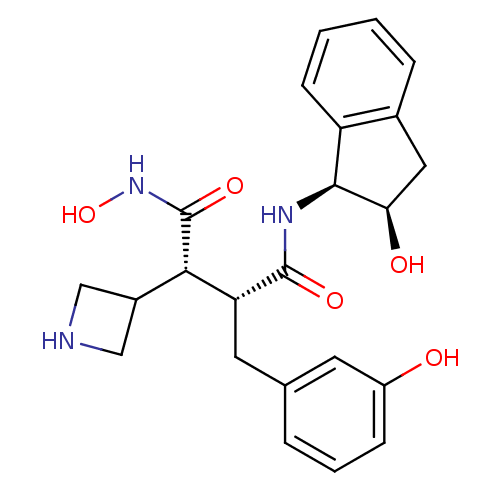 (2-Azetidin-3-yl-N*1*-hydroxy-3-((S)-3-hydroxy-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126051 (CHEMBL26388 | N*4*-Hydroxy-2-((S)-3-hydroxy-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126052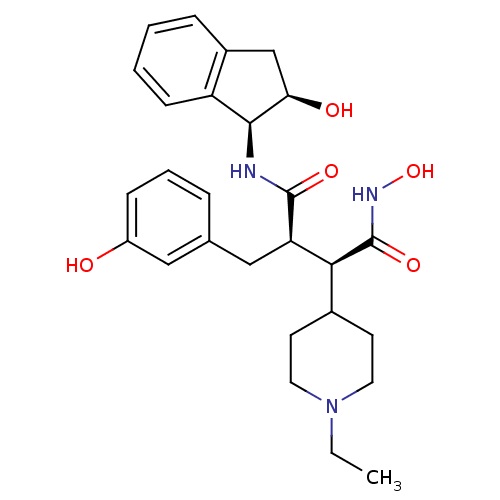 ((2R,3S)-2-(1-Ethyl-piperidin-4-yl)-N*1*-hydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126056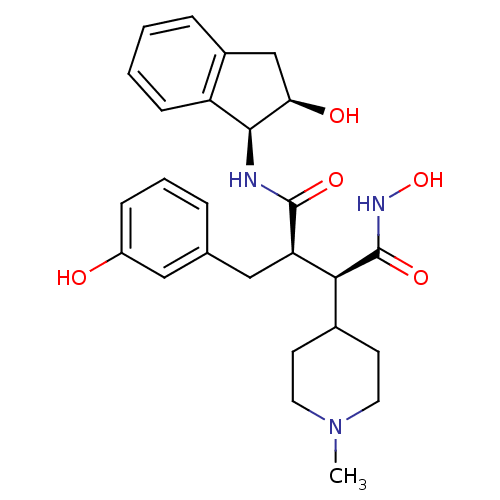 (CHEMBL24054 | N*4*-Hydroxy-2-((S)-3-hydroxy-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126050 ((2R,3S)-2-(1-Acetyl-piperidin-4-ylmethyl)-N*1*-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126048 (4-[1-Carboxy-2-((3S,3aR)-2-hydroxy-indan-1-ylcarba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50126046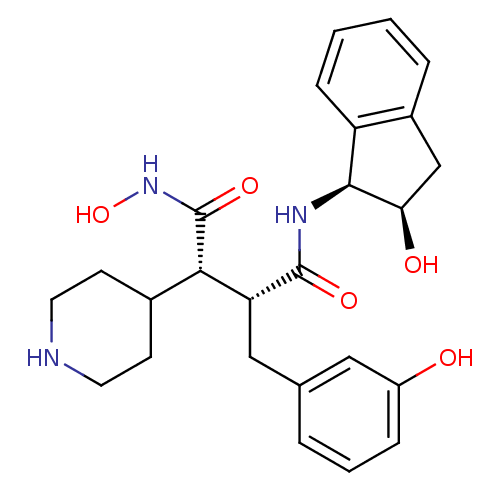 (CHEMBL24763 | N*4*-Hydroxy-2-((S)-3-hydroxy-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50104963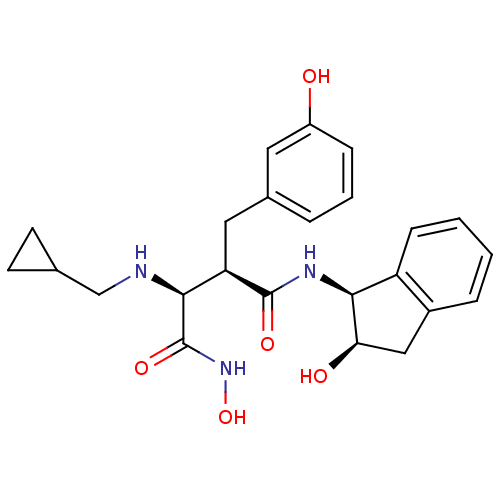 ((2S,3R)-2-(cyclopropylmethylamino)-N1-hydroxy-N4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for Matrix metalloproteinase-9(MMP-9) | Bioorg Med Chem Lett 13: 1297-300 (2003) BindingDB Entry DOI: 10.7270/Q2930SJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis | ACS Med Chem Lett 6: 1086-90 (2015) Article DOI: 10.1021/acsmedchemlett.5b00286 BindingDB Entry DOI: 10.7270/Q2ZG6W79 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50499592 (CHEMBL3741490) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis | ACS Med Chem Lett 6: 1086-90 (2015) Article DOI: 10.1021/acsmedchemlett.5b00286 BindingDB Entry DOI: 10.7270/Q2ZG6W79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234547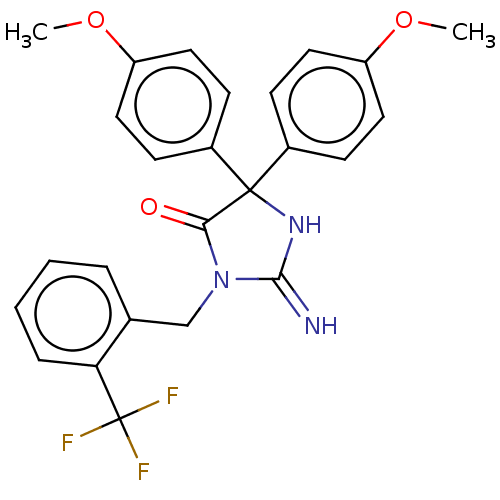 (US9353089, 304) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.88 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234542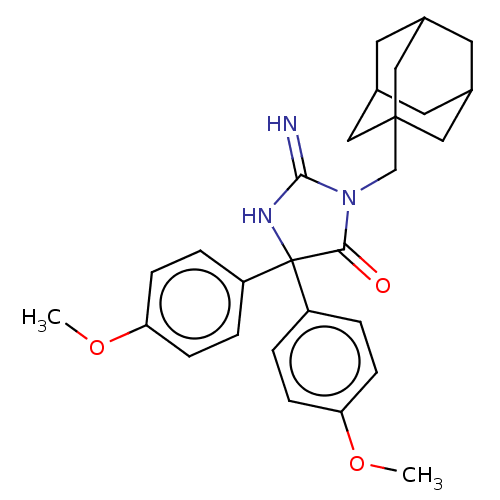 (US9353089, 276) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234527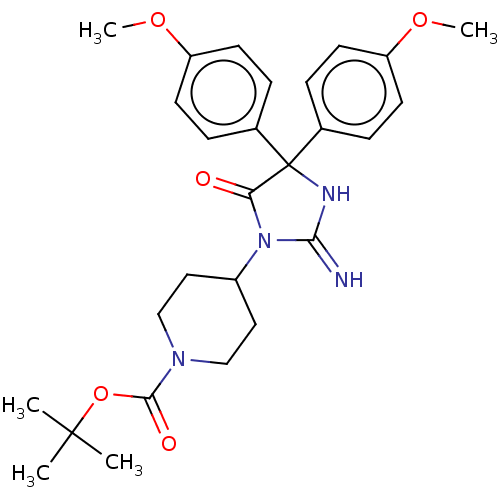 (US9353089, 206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234565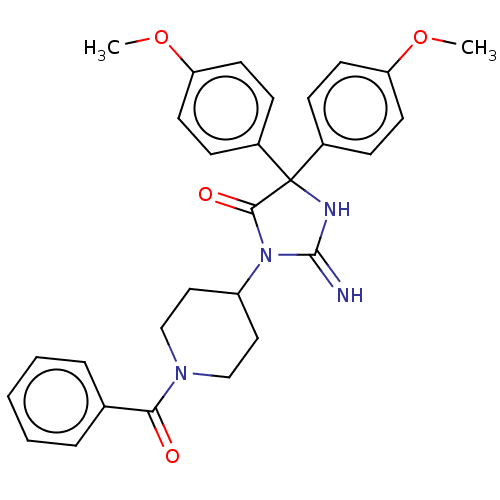 (US9353089, 311) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234537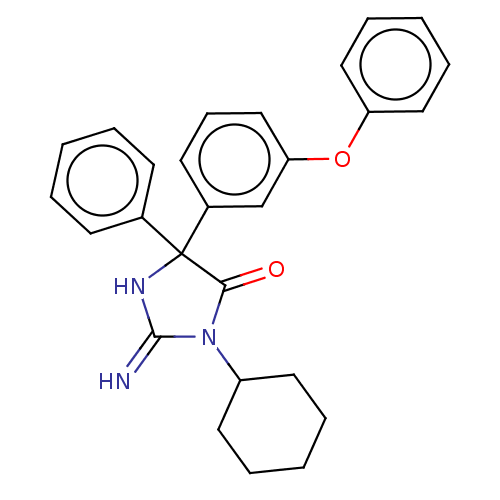 (US9353089, 238) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234534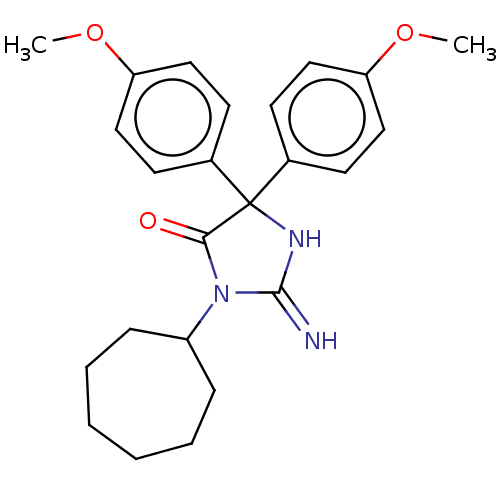 (US9353089, 220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.37 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234535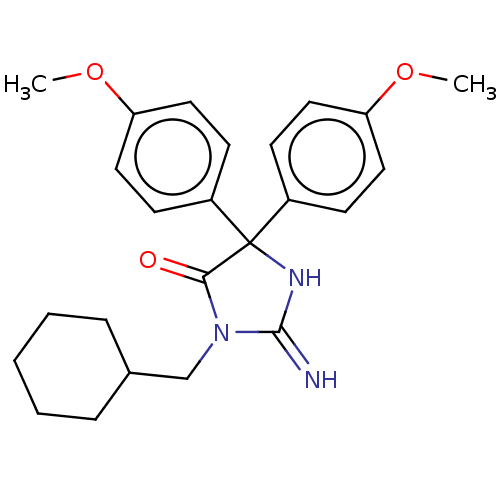 (US9353089, 221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.39 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234544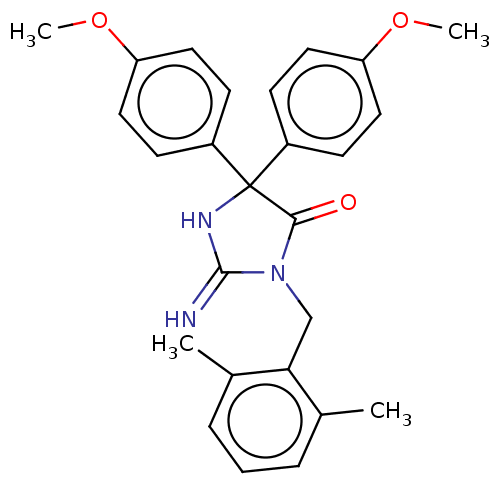 (US9353089, 278) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234527 (US9353089, 206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234536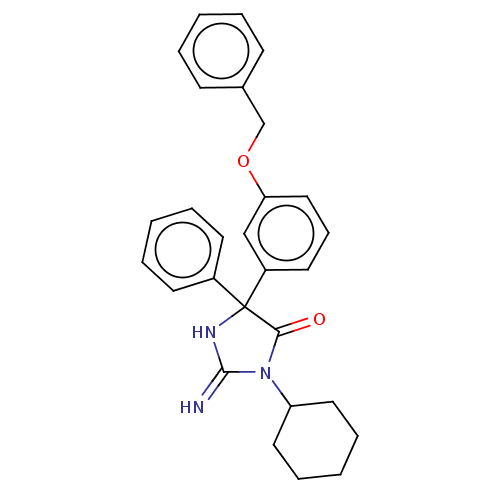 (US9353089, 228) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.89 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234546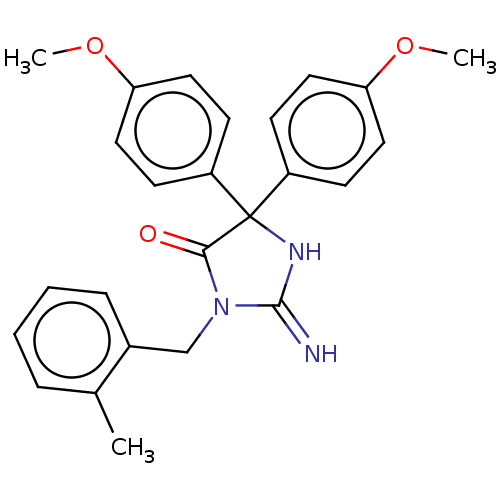 (US9353089, 303) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234497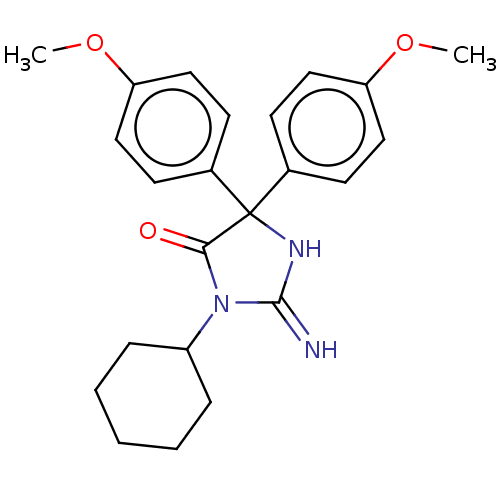 (US9353089, 117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.96 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234497 (US9353089, 117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234543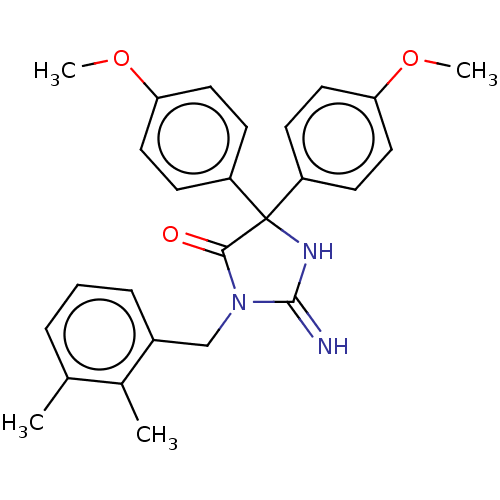 (US9353089, 277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.39 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50499586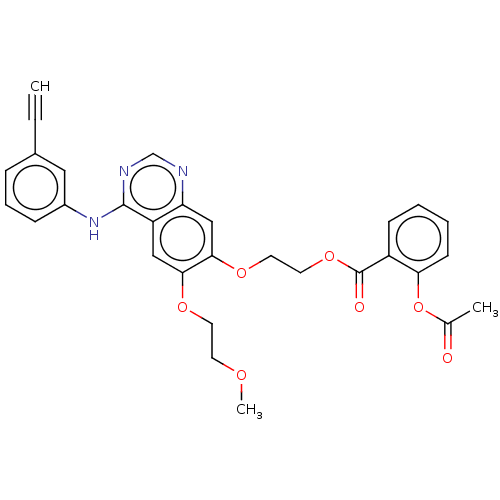 (CHEMBL3741424) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis | ACS Med Chem Lett 6: 1086-90 (2015) Article DOI: 10.1021/acsmedchemlett.5b00286 BindingDB Entry DOI: 10.7270/Q2ZG6W79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234539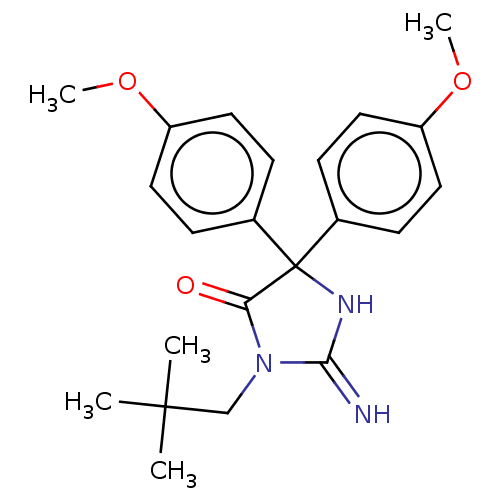 (US9353089, 252) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.28 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234529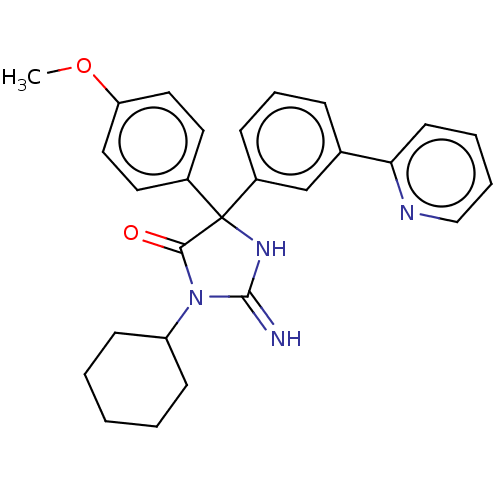 (US9353089, 210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.47 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234564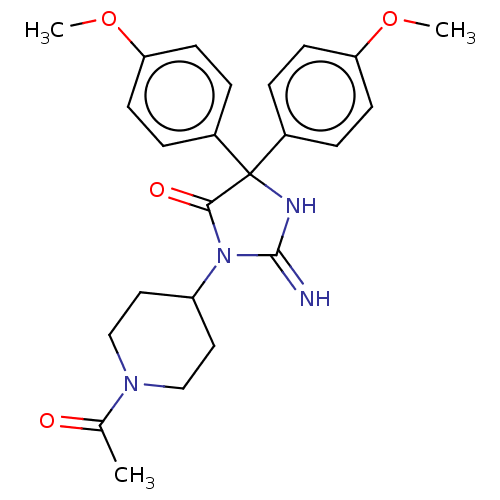 (US9353089, 310) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.84 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234508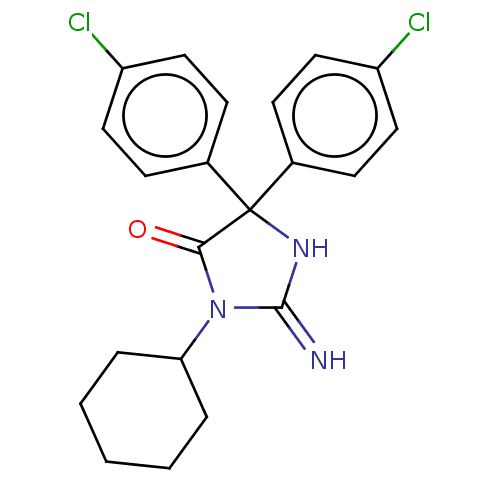 (US9353089, 155) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234520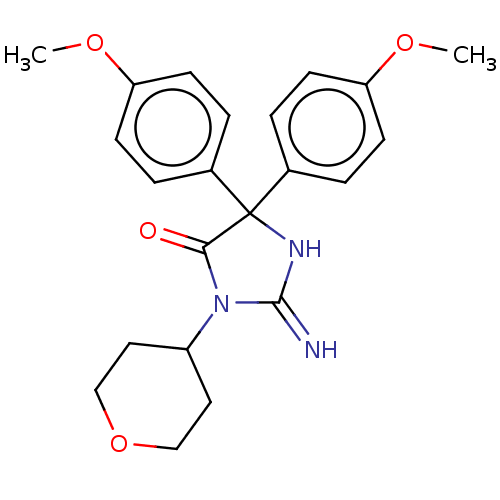 (US9353089, 176) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.81 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234510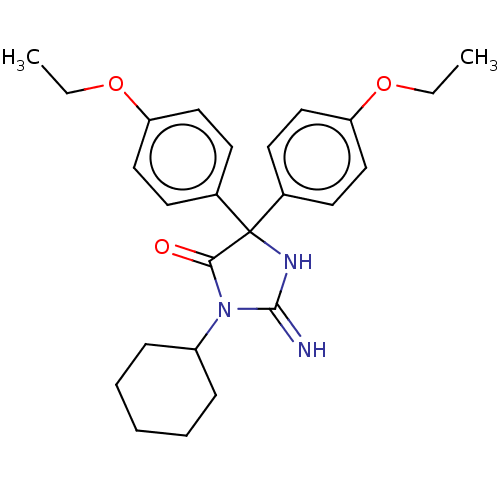 (US9353089, 157) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.87 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234517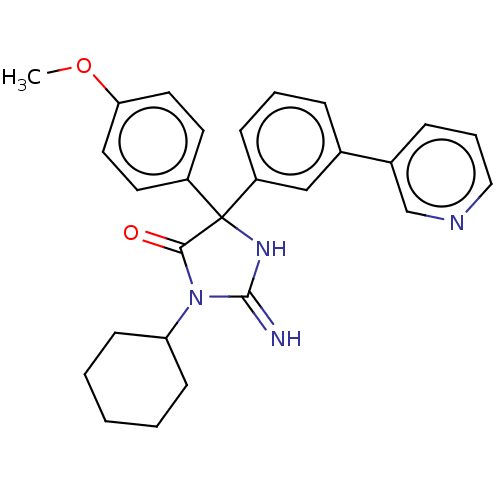 (US9353089, 171) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.97 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234520 (US9353089, 176) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin IV [1-448] (Plasmodium falciparum) | BDBM234517 (US9353089, 171) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of recombinant human COX-2 preincubated for 15 mins followed by fluorometric substrate/heme addition for 15 mins subsequently incubated wi... | J Med Chem 60: 4135-4146 (2017) Article DOI: 10.1021/acs.jmedchem.6b01484 BindingDB Entry DOI: 10.7270/Q2M32Z7Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234517 (US9353089, 171) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234513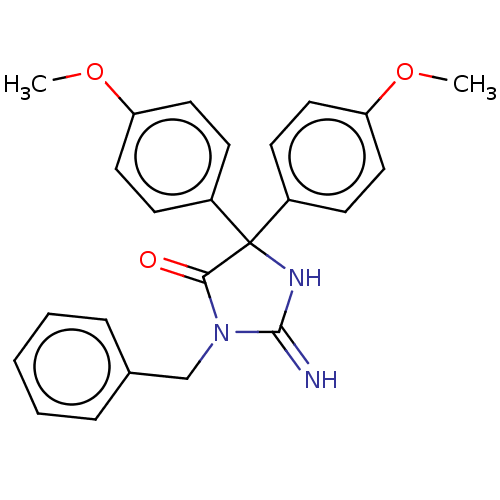 (US9353089, 162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50266867 (CHEMBL4084676) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of recombinant human COX-2 preincubated for 15 mins followed by fluorometric substrate/heme addition for 15 mins subsequently incubated wi... | J Med Chem 60: 4135-4146 (2017) Article DOI: 10.1021/acs.jmedchem.6b01484 BindingDB Entry DOI: 10.7270/Q2M32Z7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50030827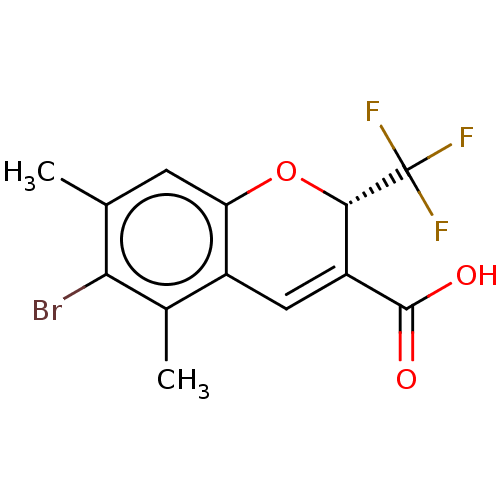 (CHEMBL3355612) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 pre-incubated for 15 mins before fluorometric substrate addition by fluorescence based assay | ACS Med Chem Lett 5: 1162-6 (2014) Article DOI: 10.1021/ml500299q BindingDB Entry DOI: 10.7270/Q23F4R87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50499595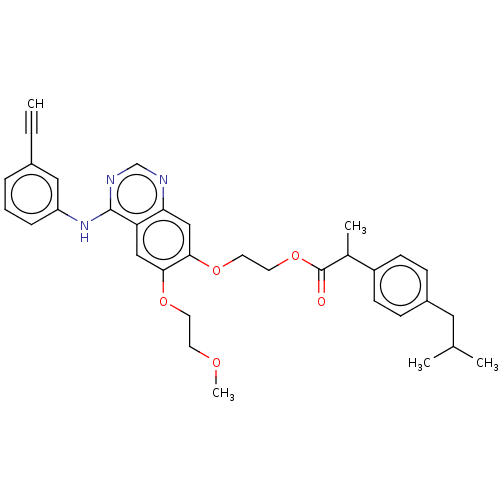 (CHEMBL3741026) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) using tyrosine 4 as substrate by fluorescence analysis | ACS Med Chem Lett 6: 1086-90 (2015) Article DOI: 10.1021/acsmedchemlett.5b00286 BindingDB Entry DOI: 10.7270/Q2ZG6W79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234567 (US9353089, 305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234533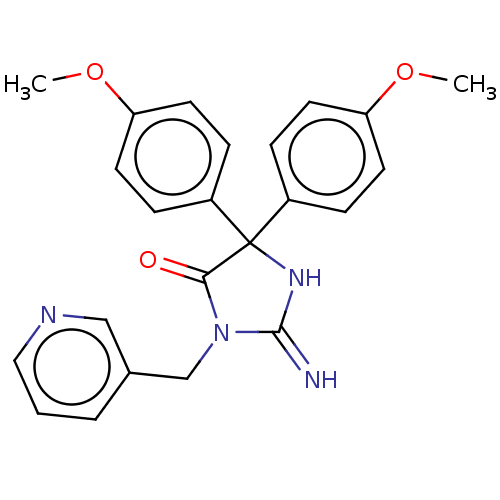 (US9353089, 219) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.62 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234518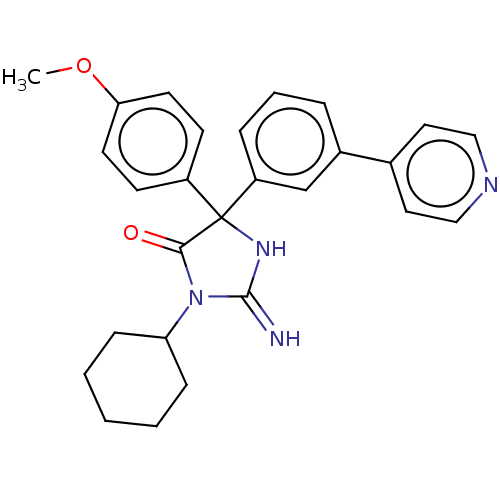 (US9353089, 172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.64 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM234507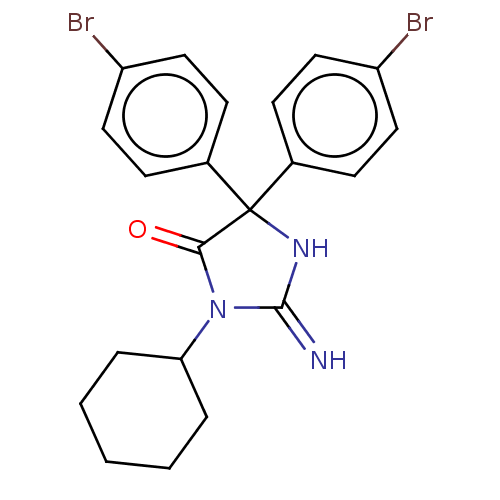 (US9353089, 154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Saint Louis University; Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences US Patent | Assay Description Plasmepsin-2 (PM-2; Plm II) and Plasmepsin (PM-4; Plm IV) expression and purification was performed following the published protocols (Istvan E S and... | US Patent US9353089 (2016) BindingDB Entry DOI: 10.7270/Q2GQ6WP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50266855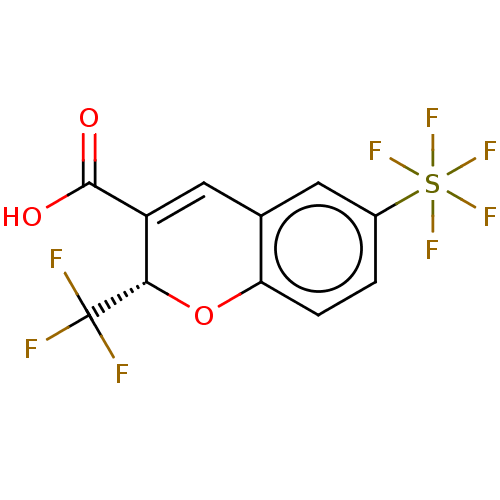 (CHEMBL4074716) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health Curated by ChEMBL | Assay Description Inhibition of recombinant human COX-2 preincubated for 15 mins followed by fluorometric substrate/heme addition for 15 mins subsequently incubated wi... | J Med Chem 60: 4135-4146 (2017) Article DOI: 10.1021/acs.jmedchem.6b01484 BindingDB Entry DOI: 10.7270/Q2M32Z7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 372 total ) | Next | Last >> |