| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 72 kDa type IV collagenase |
|---|
| Ligand | BDBM50063912 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_106804 (CHEMBL717492) |
|---|
| IC50 | 1.6±n/a nM |
|---|
| Citation |  Yamamoto, M; Tsujishita, H; Hori, N; Ohishi, Y; Inoue, S; Ikeda, S; Okada, Y Inhibition of membrane-type 1 matrix metalloproteinase by hydroxamate inhibitors: an examination of the subsite pocket. J Med Chem41:1209-17 (1998) [PubMed] Article Yamamoto, M; Tsujishita, H; Hori, N; Ohishi, Y; Inoue, S; Ikeda, S; Okada, Y Inhibition of membrane-type 1 matrix metalloproteinase by hydroxamate inhibitors: an examination of the subsite pocket. J Med Chem41:1209-17 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 72 kDa type IV collagenase |
|---|
| Name: | 72 kDa type IV collagenase |
|---|
| Synonyms: | 72 kDa gelatinase | 72 kDa type IV collagenase precursor | CLG4A | Gelatinase A | Gelatinase A (MMP-2) | MMP2 | MMP2_HUMAN | Matrix metalloproteinase-2 | Matrix metalloproteinase-2 (MMP 2) | Matrix metalloproteinase-2 (MMP2) | Matrix metalloproteinases 2 (MMP-2) | TBE-1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 73870.36 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P08253 |
|---|
| Residue: | 660 |
|---|
| Sequence: | MEALMARGALTGPLRALCLLGCLLSHAAAAPSPIIKFPGDVAPKTDKELAVQYLNTFYGC
PKESCNLFVLKDTLKKMQKFFGLPQTGDLDQNTIETMRKPRCGNPDVANYNFFPRKPKWD
KNQITYRIIGYTPDLDPETVDDAFARAFQVWSDVTPLRFSRIHDGEADIMINFGRWEHGD
GYPFDGKDGLLAHAFAPGTGVGGDSHFDDDELWTLGEGQVVRVKYGNADGEYCKFPFLFN
GKEYNSCTDTGRSDGFLWCSTTYNFEKDGKYGFCPHEALFTMGGNAEGQPCKFPFRFQGT
SYDSCTTEGRTDGYRWCGTTEDYDRDKKYGFCPETAMSTVGGNSEGAPCVFPFTFLGNKY
ESCTSAGRSDGKMWCATTANYDDDRKWGFCPDQGYSLFLVAAHEFGHAMGLEHSQDPGAL
MAPIYTYTKNFRLSQDDIKGIQELYGASPDIDLGTGPTPTLGPVTPEICKQDIVFDGIAQ
IRGEIFFFKDRFIWRTVTPRDKPMGPLLVATFWPELPEKIDAVYEAPQEEKAVFFAGNEY
WIYSASTLERGYPKPLTSLGLPPDVQRVDAAFNWSKNKKTYIFAGDKFWRYNEVKKKMDP
GFPKLIADAWNAIPDNLDAVVDLQGGGHSYFFKGAYYLKLENQSLKSVKFGSIKSDWLGC
|
|
|
|---|
| BDBM50063912 |
|---|
| n/a |
|---|
| Name | BDBM50063912 |
|---|
| Synonyms: | (R)-N*4*-Hydroxy-N*1*-((S)-methylcarbamoyl-phenyl-methyl)-2-nonyl-succinamide | CHEMBL433426 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H35N3O4 |
|---|
| Mol. Mass. | 405.531 |
|---|
| SMILES | CCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)c1ccccc1 |
|---|
| Structure | 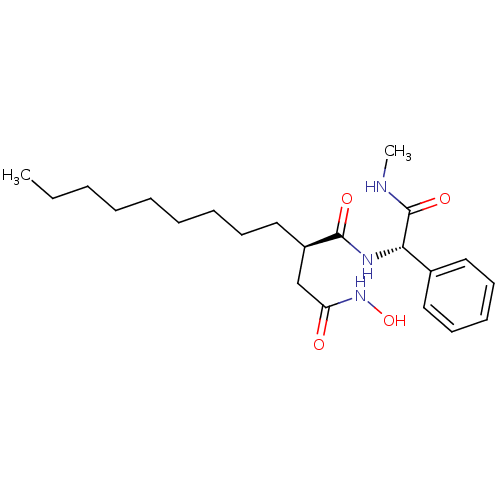
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yamamoto, M; Tsujishita, H; Hori, N; Ohishi, Y; Inoue, S; Ikeda, S; Okada, Y Inhibition of membrane-type 1 matrix metalloproteinase by hydroxamate inhibitors: an examination of the subsite pocket. J Med Chem41:1209-17 (1998) [PubMed] Article
Yamamoto, M; Tsujishita, H; Hori, N; Ohishi, Y; Inoue, S; Ikeda, S; Okada, Y Inhibition of membrane-type 1 matrix metalloproteinase by hydroxamate inhibitors: an examination of the subsite pocket. J Med Chem41:1209-17 (1998) [PubMed] Article