 Found 1058 hits with Last Name = 'yamamoto' and Initial = 'm'
Found 1058 hits with Last Name = 'yamamoto' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109635
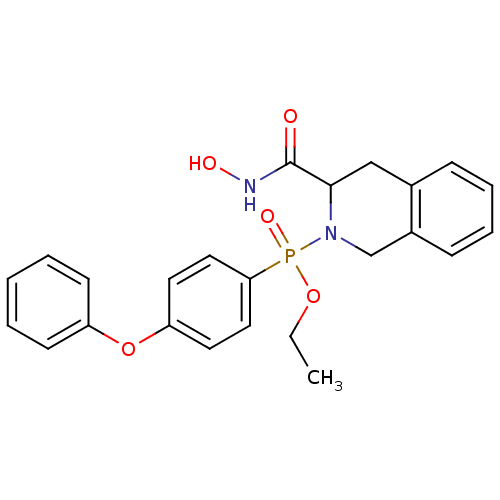
((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H25N2O5P/c1-2-30-32(29,22-14-12-21(13-15-22)31-20-10-4-3-5-11-20)26-17-19-9-7-6-8-18(19)16-23(26)24(27)25-28/h3-15,23,28H,2,16-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50547388
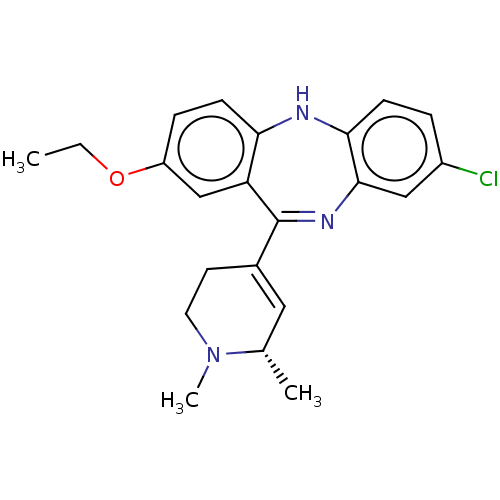
(CHEMBL4758966)Show SMILES CCOc1ccc2Nc3ccc(Cl)cc3N=C(C3=C[C@H](C)N(C)CC3)c2c1 |r,t:16,18| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5HT2A receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50547385
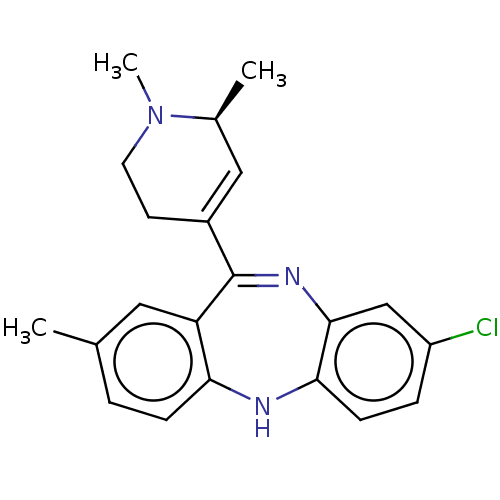
(CHEMBL4745124)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2cc(Cl)ccc2Nc2ccc(C)cc12 |r,c:2,t:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5HT2A receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109621
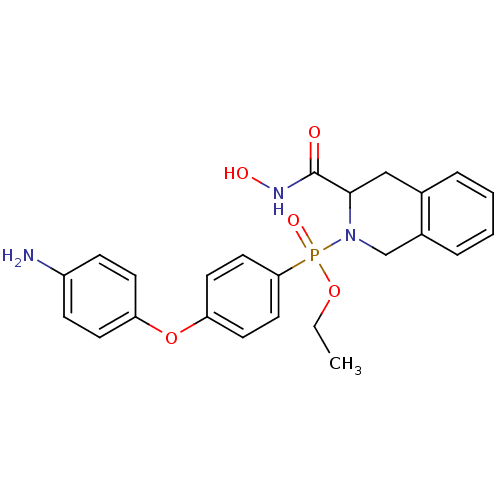
(CHEMBL425316 | [4-(4-Amino-phenoxy)-phenyl]-(3-hyd...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccc(N)cc2)cc1 Show InChI InChI=1S/C24H26N3O5P/c1-2-31-33(30,22-13-11-21(12-14-22)32-20-9-7-19(25)8-10-20)27-16-18-6-4-3-5-17(18)15-23(27)24(28)26-29/h3-14,23,29H,2,15-16,25H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547397
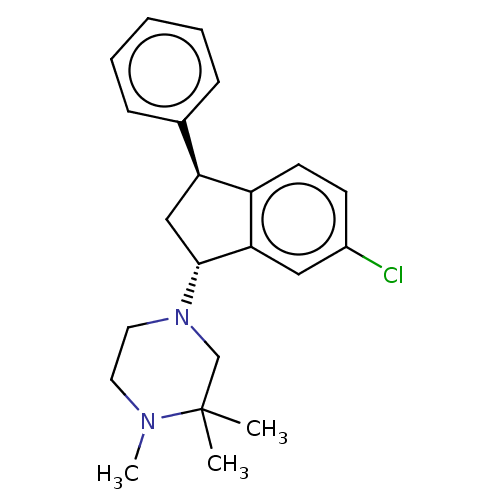
(LU-31-130 | Lu 31-130 | Zicronapine)Show SMILES CN1CCN(CC1(C)C)[C@@H]1C[C@H](c2ccc(Cl)cc12)c1ccccc1 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50547389
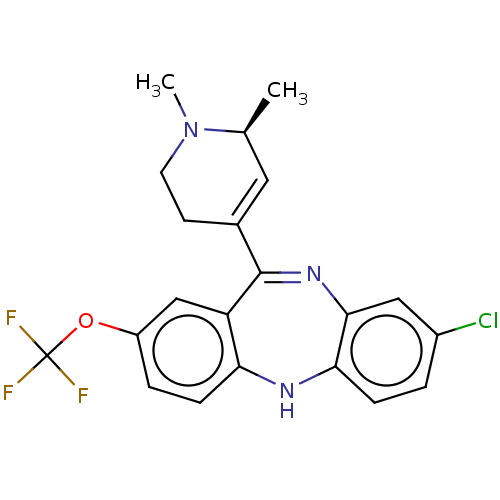
(CHEMBL4745489)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2cc(Cl)ccc2Nc2ccc(OC(F)(F)F)cc12 |r,c:2,t:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5HT2A receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50547386

(CHEMBL4760903)Show SMILES CCc1ccc2Nc3ccc(Cl)cc3N=C(C3=C[C@H](C)N(C)CC3)c2c1 |r,t:15,17| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5HT2A receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547390
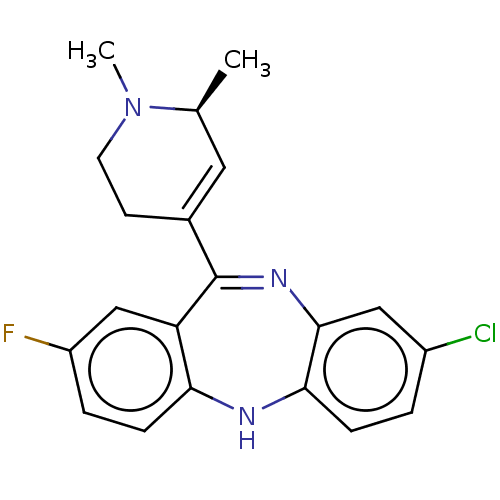
(CHEMBL4758723)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2cc(Cl)ccc2Nc2ccc(F)cc12 |r,c:2,t:9| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109626
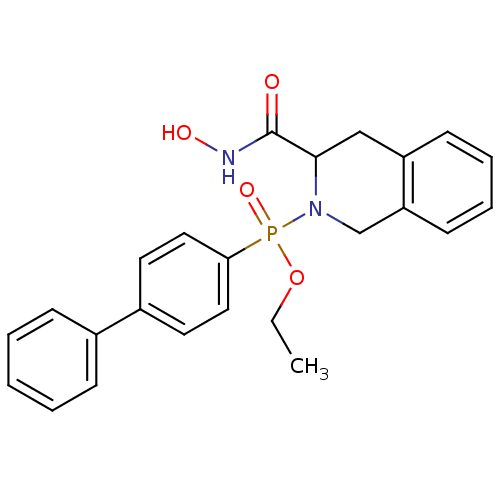
(Biphenyl-4-yl-(3-hydroxycarbamoyl-3,4-dihydro-1H-i...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C24H25N2O4P/c1-2-30-31(29,22-14-12-19(13-15-22)18-8-4-3-5-9-18)26-17-21-11-7-6-10-20(21)16-23(26)24(27)25-28/h3-15,23,28H,2,16-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5HT2A receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547394
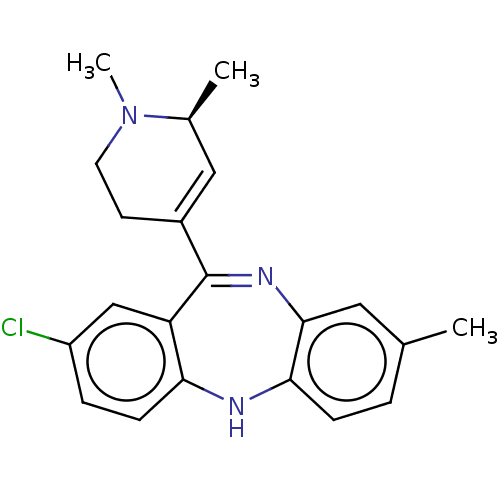
(CHEMBL4756254)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2cc(C)ccc2Nc2ccc(Cl)cc12 |r,c:2,t:9| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109621

(CHEMBL425316 | [4-(4-Amino-phenoxy)-phenyl]-(3-hyd...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccc(N)cc2)cc1 Show InChI InChI=1S/C24H26N3O5P/c1-2-31-33(30,22-13-11-21(12-14-22)32-20-9-7-19(25)8-10-20)27-16-18-6-4-3-5-17(18)15-23(27)24(28)26-29/h3-14,23,29H,2,15-16,25H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109635

((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H25N2O5P/c1-2-30-32(29,22-14-12-21(13-15-22)31-20-10-4-3-5-11-20)26-17-19-9-7-6-8-18(19)16-23(26)24(27)25-28/h3-15,23,28H,2,16-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109625
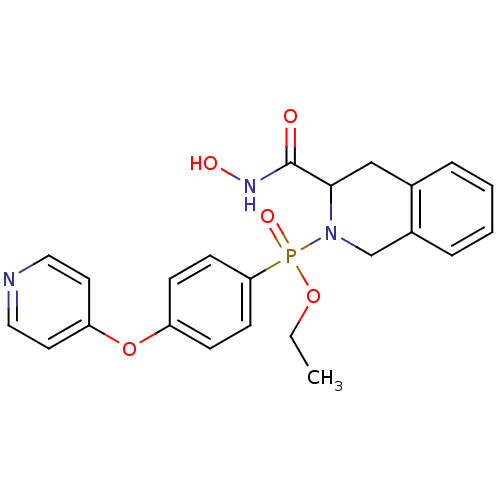
((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C23H24N3O5P/c1-2-30-32(29,21-9-7-19(8-10-21)31-20-11-13-24-14-12-20)26-16-18-6-4-3-5-17(18)15-22(26)23(27)25-28/h3-14,22,28H,2,15-16H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547391
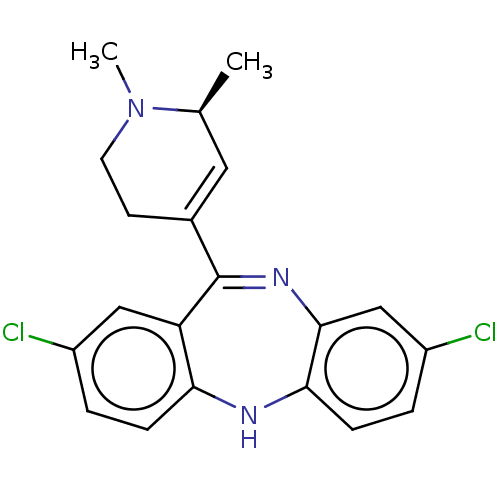
(CHEMBL4795230)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2cc(Cl)ccc2Nc2ccc(Cl)cc12 |r,c:2,t:9| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004823
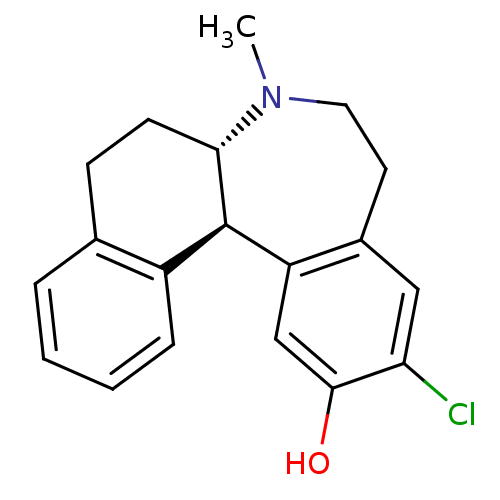
((6aS,13bR)-11-Chloro-7-methyl-5,6a,7,8,9,13b-hexah...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1ccccc21 Show InChI InChI=1S/C19H20ClNO/c1-21-9-8-13-10-16(20)18(22)11-15(13)19-14-5-3-2-4-12(14)6-7-17(19)21/h2-5,10-11,17,19,22H,6-9H2,1H3/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D1 |
J Med Chem 34: 2946-53 (1991)
BindingDB Entry DOI: 10.7270/Q2H132MN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50109629
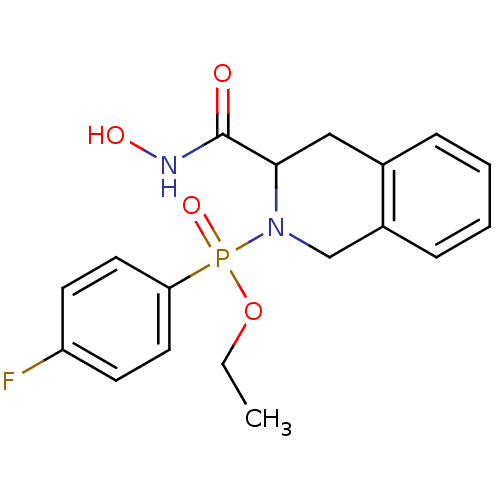
((4-Fluoro-phenyl)-(3-hydroxycarbamoyl-3,4-dihydro-...)Show InChI InChI=1S/C18H20FN2O4P/c1-2-25-26(24,16-9-7-15(19)8-10-16)21-12-14-6-4-3-5-13(14)11-17(21)18(22)20-23/h3-10,17,23H,2,11-12H2,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-1 (MMP-1)(recombinant human collagenase-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547392

(CHEMBL4742056)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2cc(Cl)ccc2Nc2ccc(cc12)C#N |r,c:2,t:9| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109633
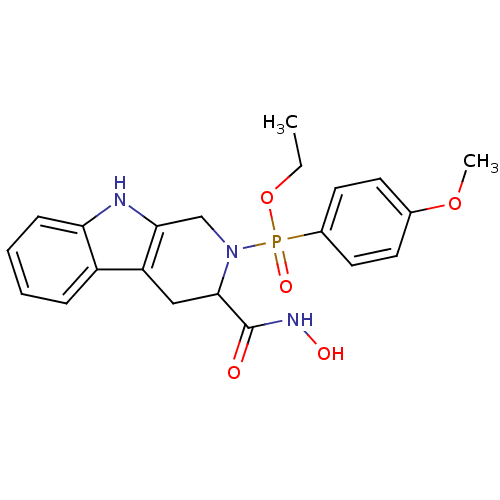
((3-Hydroxycarbamoyl-1,3,4,9-tetrahydro-beta-carbol...)Show SMILES CCOP(=O)(N1Cc2[nH]c3ccccc3c2CC1C(=O)NO)c1ccc(OC)cc1 Show InChI InChI=1S/C21H24N3O5P/c1-3-29-30(27,15-10-8-14(28-2)9-11-15)24-13-19-17(12-20(24)21(25)23-26)16-6-4-5-7-18(16)22-19/h4-11,20,22,26H,3,12-13H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM84958

(2-[[(R)-2-(1H-Indol-2-ylcarbonylamino)-3-(4-benzhy...)Show SMILES OC(=O)c1cccnc1SC[C@H](NC(=O)c1cc2ccccc2[nH]1)C(=O)N1CCN(CC1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H33N5O4S/c41-32(29-22-26-14-7-8-16-28(26)37-29)38-30(23-45-33-27(35(43)44)15-9-17-36-33)34(42)40-20-18-39(19-21-40)31(24-10-3-1-4-11-24)25-12-5-2-6-13-25/h1-17,22,30-31,37H,18-21,23H2,(H,38,41)(H,43,44)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
D(1) dopamine receptor
(Carassius auratus) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of dopamine stimulated adenylate cyclase |
J Med Chem 34: 2946-53 (1991)
BindingDB Entry DOI: 10.7270/Q2H132MN |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547386

(CHEMBL4760903)Show SMILES CCc1ccc2Nc3ccc(Cl)cc3N=C(C3=C[C@H](C)N(C)CC3)c2c1 |r,t:15,17| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5HT2A receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547396
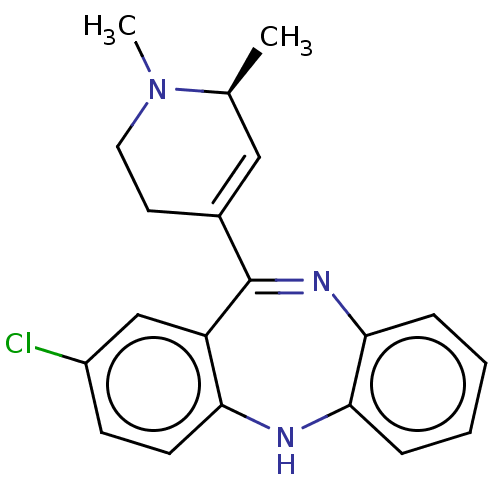
(CHEMBL4740880)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2ccccc2Nc2ccc(Cl)cc12 |r,c:2,t:9| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50109635

((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H25N2O5P/c1-2-30-32(29,22-14-12-21(13-15-22)31-20-10-4-3-5-11-20)26-17-19-9-7-6-8-18(19)16-23(26)24(27)25-28/h3-15,23,28H,2,16-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-1 (MMP-1)(recombinant human collagenase-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547389

(CHEMBL4745489)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2cc(Cl)ccc2Nc2ccc(OC(F)(F)F)cc12 |r,c:2,t:9| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547387

(CHEMBL4757297)Show SMILES COc1ccc2Nc3ccc(Cl)cc3N=C(C3=C[C@H](C)N(C)CC3)c2c1 |r,t:15,17| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50109633

((3-Hydroxycarbamoyl-1,3,4,9-tetrahydro-beta-carbol...)Show SMILES CCOP(=O)(N1Cc2[nH]c3ccccc3c2CC1C(=O)NO)c1ccc(OC)cc1 Show InChI InChI=1S/C21H24N3O5P/c1-3-29-30(27,15-10-8-14(28-2)9-11-15)24-13-19-17(12-20(24)21(25)23-26)16-6-4-5-7-18(16)22-19/h4-11,20,22,26H,3,12-13H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-1 (MMP-1)(recombinant human collagenase-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109630
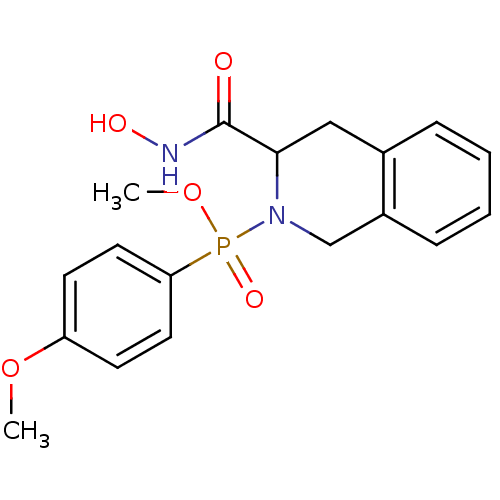
((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show InChI InChI=1S/C18H21N2O5P/c1-24-15-7-9-16(10-8-15)26(23,25-2)20-12-14-6-4-3-5-13(14)11-17(20)18(21)19-22/h3-10,17,22H,11-12H2,1-2H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109633

((3-Hydroxycarbamoyl-1,3,4,9-tetrahydro-beta-carbol...)Show SMILES CCOP(=O)(N1Cc2[nH]c3ccccc3c2CC1C(=O)NO)c1ccc(OC)cc1 Show InChI InChI=1S/C21H24N3O5P/c1-3-29-30(27,15-10-8-14(28-2)9-11-15)24-13-19-17(12-20(24)21(25)23-26)16-6-4-5-7-18(16)22-19/h4-11,20,22,26H,3,12-13H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50109633

((3-Hydroxycarbamoyl-1,3,4,9-tetrahydro-beta-carbol...)Show SMILES CCOP(=O)(N1Cc2[nH]c3ccccc3c2CC1C(=O)NO)c1ccc(OC)cc1 Show InChI InChI=1S/C21H24N3O5P/c1-3-29-30(27,15-10-8-14(28-2)9-11-15)24-13-19-17(12-20(24)21(25)23-26)16-6-4-5-7-18(16)22-19/h4-11,20,22,26H,3,12-13H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tumor necrosis factor alpha converting enzyme (TACE) from human acute monocytic leukemia cell line. |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547385

(CHEMBL4745124)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2cc(Cl)ccc2Nc2ccc(C)cc12 |r,c:2,t:9| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547405
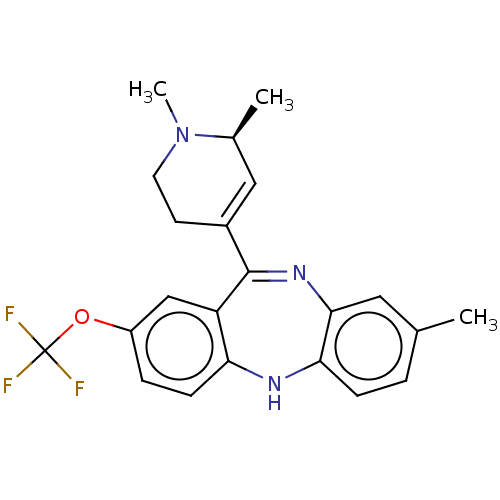
(CHEMBL4797375)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2cc(C)ccc2Nc2ccc(OC(F)(F)F)cc12 |r,c:2,t:9| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109622

((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES COc1ccc(cc1)P(=O)(OCC1CCCCC1)N1Cc2ccccc2CC1C(=O)NO Show InChI InChI=1S/C24H31N2O5P/c1-30-21-11-13-22(14-12-21)32(29,31-17-18-7-3-2-4-8-18)26-16-20-10-6-5-9-19(20)15-23(26)24(27)25-28/h5-6,9-14,18,23,28H,2-4,7-8,15-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109630

((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show InChI InChI=1S/C18H21N2O5P/c1-24-15-7-9-16(10-8-15)26(23,25-2)20-12-14-6-4-3-5-13(14)11-17(20)18(21)19-22/h3-10,17,22H,11-12H2,1-2H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50109614

(CHEMBL353536 | [4-(2-Ethoxy-ethoxy)-phenyl]-(3-hyd...)Show SMILES CCOCCOc1ccc(cc1)P(=O)(OCC)N1Cc2ccccc2CC1C(=O)NO Show InChI InChI=1S/C22H29N2O6P/c1-3-28-13-14-29-19-9-11-20(12-10-19)31(27,30-4-2)24-16-18-8-6-5-7-17(18)15-21(24)22(25)23-26/h5-12,21,26H,3-4,13-16H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tumor necrosis factor alpha converting enzyme (TACE) from human acute monocytic leukemia cell line. |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50007129

((1R,3S)-1-Aminomethyl-3-phenyl-isochroman-5,6-diol...)Show InChI InChI=1S/C16H17NO3/c17-9-15-11-6-7-13(18)16(19)12(11)8-14(20-15)10-4-2-1-3-5-10/h1-7,14-15,18-19H,8-9,17H2/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D1 |
J Med Chem 34: 2946-53 (1991)
BindingDB Entry DOI: 10.7270/Q2H132MN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50109631
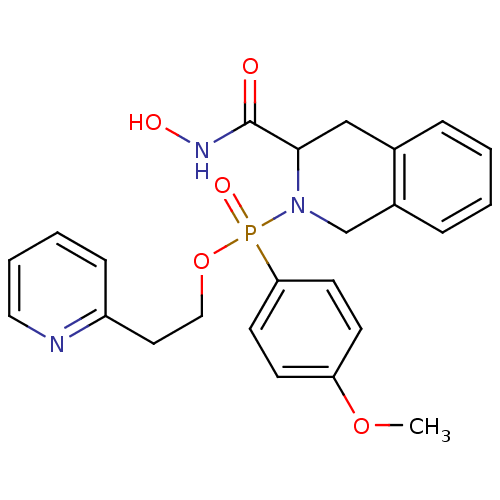
((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES COc1ccc(cc1)P(=O)(OCCc1ccccn1)N1Cc2ccccc2CC1C(=O)NO Show InChI InChI=1S/C24H26N3O5P/c1-31-21-9-11-22(12-10-21)33(30,32-15-13-20-8-4-5-14-25-20)27-17-19-7-3-2-6-18(19)16-23(27)24(28)26-29/h2-12,14,23,29H,13,15-17H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-1 (MMP-1)(recombinant human collagenase-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109631

((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES COc1ccc(cc1)P(=O)(OCCc1ccccn1)N1Cc2ccccc2CC1C(=O)NO Show InChI InChI=1S/C24H26N3O5P/c1-31-21-9-11-22(12-10-21)33(30,32-15-13-20-8-4-5-14-25-20)27-17-19-7-3-2-6-18(19)16-23(27)24(28)26-29/h2-12,14,23,29H,13,15-17H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50109630

((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show InChI InChI=1S/C18H21N2O5P/c1-24-15-7-9-16(10-8-15)26(23,25-2)20-12-14-6-4-3-5-13(14)11-17(20)18(21)19-22/h3-10,17,22H,11-12H2,1-2H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-1 (MMP-1)(recombinant human collagenase-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547388

(CHEMBL4758966)Show SMILES CCOc1ccc2Nc3ccc(Cl)cc3N=C(C3=C[C@H](C)N(C)CC3)c2c1 |r,t:16,18| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109624
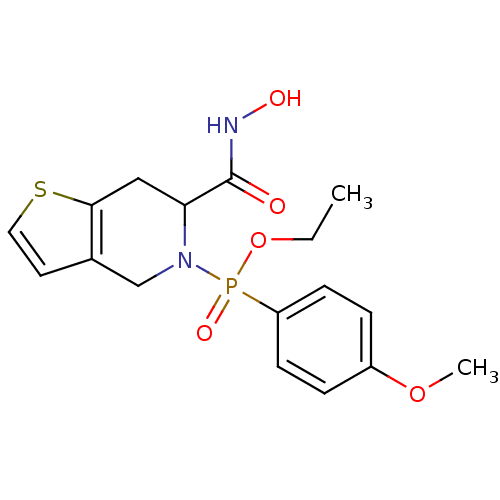
((6-Hydroxycarbamoyl-6,7-dihydro-4H-thieno[3,2-c]py...)Show InChI InChI=1S/C17H21N2O5PS/c1-3-24-25(22,14-6-4-13(23-2)5-7-14)19-11-12-8-9-26-16(12)10-15(19)17(20)18-21/h4-9,15,21H,3,10-11H2,1-2H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50547395
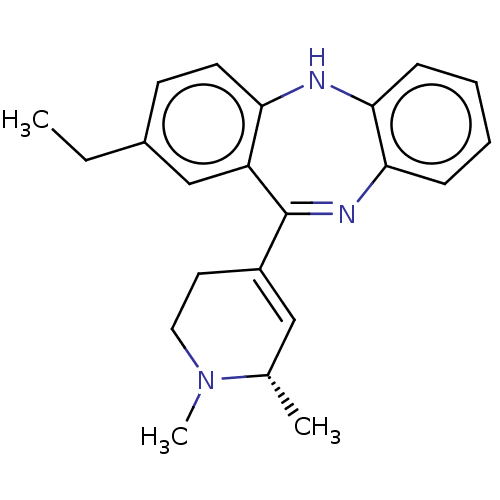
(CHEMBL4756987)Show SMILES CCc1ccc2Nc3ccccc3N=C(C3=C[C@H](C)N(C)CC3)c2c1 |r,t:14,16| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human dopamine D1 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50109624

((6-Hydroxycarbamoyl-6,7-dihydro-4H-thieno[3,2-c]py...)Show InChI InChI=1S/C17H21N2O5PS/c1-3-24-25(22,14-6-4-13(23-2)5-7-14)19-11-12-8-9-26-16(12)10-15(19)17(20)18-21/h4-9,15,21H,3,10-11H2,1-2H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-1 (MMP-1)(recombinant human collagenase-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109626

(Biphenyl-4-yl-(3-hydroxycarbamoyl-3,4-dihydro-1H-i...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C24H25N2O4P/c1-2-30-31(29,22-14-12-19(13-15-22)18-8-4-3-5-9-18)26-17-21-11-7-6-10-20(21)16-23(26)24(27)25-28/h3-15,23,28H,2,16-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50109618
((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES COc1ccc(cc1)P(=O)(OCCc1ccccc1)N1Cc2ccccc2CC1C(=O)NO Show InChI InChI=1S/C25H27N2O5P/c1-31-22-11-13-23(14-12-22)33(30,32-16-15-19-7-3-2-4-8-19)27-18-21-10-6-5-9-20(21)17-24(27)25(28)26-29/h2-14,24,29H,15-18H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-1 (MMP-1)(recombinant human collagenase-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50109622

((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES COc1ccc(cc1)P(=O)(OCC1CCCCC1)N1Cc2ccccc2CC1C(=O)NO Show InChI InChI=1S/C24H31N2O5P/c1-30-21-11-13-22(14-12-21)32(29,31-17-18-7-3-2-4-8-18)26-16-20-10-6-5-9-19(20)15-23(26)24(27)25-28/h5-6,9-14,18,23,28H,2-4,7-8,15-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP-9)(gelatinase-B). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data