| Reaction Details |
|---|
 | Report a problem with these data |
| Target | cAMP-specific 3',5'-cyclic phosphodiesterase 4B |
|---|
| Ligand | BDBM50453158 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1849722 (CHEMBL4350263) |
|---|
| Ki | 1000±n/a nM |
|---|
| Citation |  de Heuvel, E; Singh, AK; Edink, E; van der Meer, T; van der Woude, M; Sadek, P; Krell-Jørgensen, MP; van den Bergh, T; Veerman, J; Caljon, G; Kalejaiye, TD; Wijtmans, M; Maes, L; de Koning, HP; Jan Sterk, G; Siderius, M; de Esch, IJP; Brown, DG; Leurs, R Alkynamide phthalazinones as a new class of TbrPDEB1 inhibitors. Bioorg Med Chem27:3998-4012 (2019) [PubMed] Article de Heuvel, E; Singh, AK; Edink, E; van der Meer, T; van der Woude, M; Sadek, P; Krell-Jørgensen, MP; van den Bergh, T; Veerman, J; Caljon, G; Kalejaiye, TD; Wijtmans, M; Maes, L; de Koning, HP; Jan Sterk, G; Siderius, M; de Esch, IJP; Brown, DG; Leurs, R Alkynamide phthalazinones as a new class of TbrPDEB1 inhibitors. Bioorg Med Chem27:3998-4012 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B |
|---|
| Name: | cAMP-specific 3',5'-cyclic phosphodiesterase 4B |
|---|
| Synonyms: | 3',5'-cyclic phosphodiesterase | DPDE4 | Isoform PDE4B1 | PDE32 | PDE4B | PDE4B1 | PDE4B_HUMAN | Phosphodiesterase 4B | Phosphodiesterase 4B (PDE4B) | Phosphodiesterase 4B (PDE4B1) | Phosphodiesterase Type 4 (PDE4B) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 83318.87 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q07343 |
|---|
| Residue: | 736 |
|---|
| Sequence: | MKKSRSVMTVMADDNVKDYFECSLSKSYSSSSNTLGIDLWRGRRCCSGNLQLPPLSQRQS
ERARTPEGDGISRPTTLPLTTLPSIAITTVSQECFDVENGPSPGRSPLDPQASSSAGLVL
HATFPGHSQRRESFLYRSDSDYDLSPKAMSRNSSLPSEQHGDDLIVTPFAQVLASLRSVR
NNFTILTNLHGTSNKRSPAASQPPVSRVNPQEESYQKLAMETLEELDWCLDQLETIQTYR
SVSEMASNKFKRMLNRELTHLSEMSRSGNQVSEYISNTFLDKQNDVEIPSPTQKDREKKK
KQQLMTQISGVKKLMHSSSLNNTSISRFGVNTENEDHLAKELEDLNKWGLNIFNVAGYSH
NRPLTCIMYAIFQERDLLKTFRISSDTFITYMMTLEDHYHSDVAYHNSLHAADVAQSTHV
LLSTPALDAVFTDLEILAAIFAAAIHDVDHPGVSNQFLINTNSELALMYNDESVLENHHL
AVGFKLLQEEHCDIFMNLTKKQRQTLRKMVIDMVLATDMSKHMSLLADLKTMVETKKVTS
SGVLLLDNYTDRIQVLRNMVHCADLSNPTKSLELYRQWTDRIMEEFFQQGDKERERGMEI
SPMCDKHTASVEKSQVGFIDYIVHPLWETWADLVQPDAQDILDTLEDNRNWYQSMIPQSP
SPPLDEQNRDCQGLMEKFQFELTLDEEDSEGPEKEGEGHSYFSSTKTLCVIDPENRDSLG
ETDIDIATEDKSPVDT
|
|
|
|---|
| BDBM50453158 |
|---|
| n/a |
|---|
| Name | BDBM50453158 |
|---|
| Synonyms: | CHEMBL4210475 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H36N4O4 |
|---|
| Mol. Mass. | 528.6419 |
|---|
| SMILES | [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCCCCC1)C2=O)c1ccc(OC)c(c1)-c1ccc(cc1)C(=O)NCC(N)=O |r,c:3,9| |
|---|
| Structure | 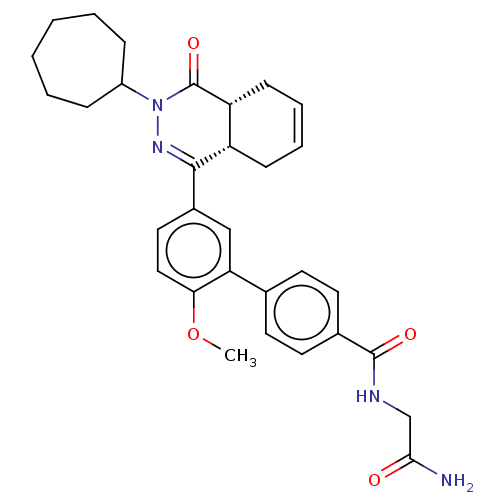
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 de Heuvel, E; Singh, AK; Edink, E; van der Meer, T; van der Woude, M; Sadek, P; Krell-Jørgensen, MP; van den Bergh, T; Veerman, J; Caljon, G; Kalejaiye, TD; Wijtmans, M; Maes, L; de Koning, HP; Jan Sterk, G; Siderius, M; de Esch, IJP; Brown, DG; Leurs, R Alkynamide phthalazinones as a new class of TbrPDEB1 inhibitors. Bioorg Med Chem27:3998-4012 (2019) [PubMed] Article
de Heuvel, E; Singh, AK; Edink, E; van der Meer, T; van der Woude, M; Sadek, P; Krell-Jørgensen, MP; van den Bergh, T; Veerman, J; Caljon, G; Kalejaiye, TD; Wijtmans, M; Maes, L; de Koning, HP; Jan Sterk, G; Siderius, M; de Esch, IJP; Brown, DG; Leurs, R Alkynamide phthalazinones as a new class of TbrPDEB1 inhibitors. Bioorg Med Chem27:3998-4012 (2019) [PubMed] Article