 Found 2508 hits with Last Name = 'leurs' and Initial = 'r'
Found 2508 hits with Last Name = 'leurs' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50397290
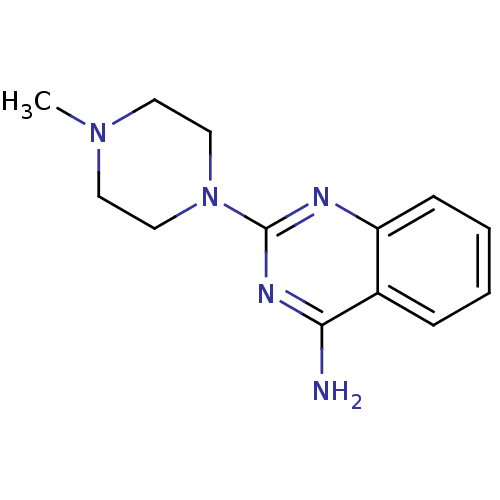
(CHEMBL475331 | VUF-10147)Show InChI InChI=1S/C13H17N5/c1-17-6-8-18(9-7-17)13-15-11-5-3-2-4-10(11)12(14)16-13/h2-5H,6-9H2,1H3,(H2,14,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from 5HT3A receptor expressed in HEK293 cells after 24 hrs by scintillation counting in presence of quipazine |
J Med Chem 55: 8603-14 (2012)
Article DOI: 10.1021/jm300801u
BindingDB Entry DOI: 10.7270/Q2RF5W5T |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527524

(CHEMBL4566742)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CCC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H32N4O6/c1-36-21-8-7-17(15-22(21)37-2)26-19-5-3-4-6-20(19)27(35)31(28-26)18-11-13-29(14-12-18)25(34)16-30-23(32)9-10-24(30)33/h3-4,7-8,15,18-20H,5-6,9-14,16H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50079527
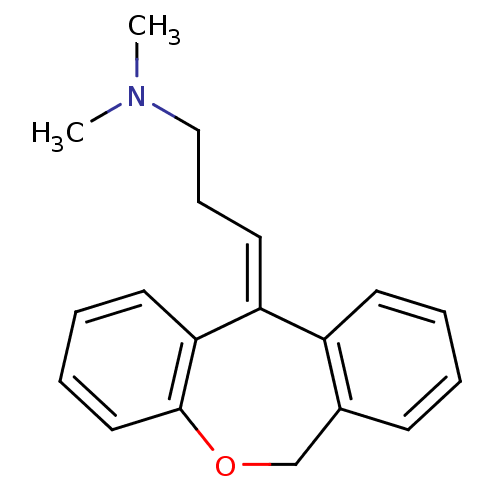
((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 328-36 (2002)
Article DOI: 10.1124/jpet.302.1.328
BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527552
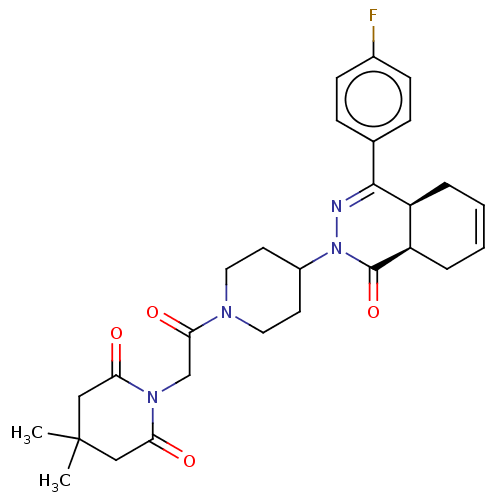
(CHEMBL4435111)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(F)cc1 |r,c:3,9| Show InChI InChI=1S/C28H33FN4O4/c1-28(2)15-23(34)32(24(35)16-28)17-25(36)31-13-11-20(12-14-31)33-27(37)22-6-4-3-5-21(22)26(30-33)18-7-9-19(29)10-8-18/h3-4,7-10,20-22H,5-6,11-17H2,1-2H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527532
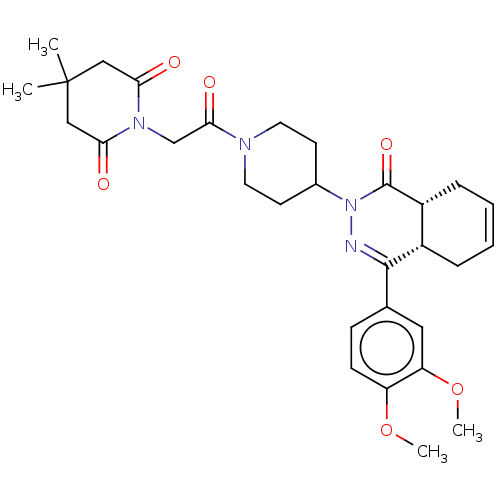
(CHEMBL4441748)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C30H38N4O6/c1-30(2)16-25(35)33(26(36)17-30)18-27(37)32-13-11-20(12-14-32)34-29(38)22-8-6-5-7-21(22)28(31-34)19-9-10-23(39-3)24(15-19)40-4/h5-6,9-10,15,20-22H,7-8,11-14,16-18H2,1-4H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527537
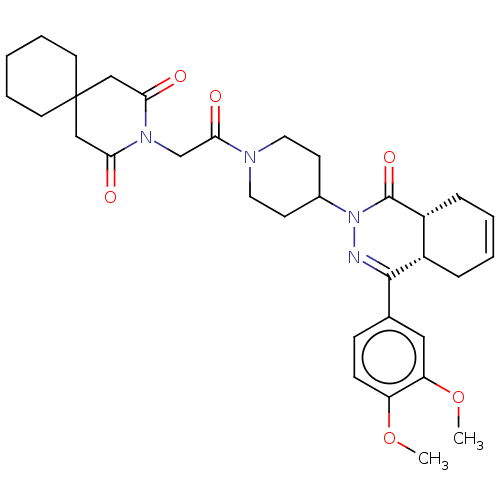
(CHEMBL4453005)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC3(CCCCC3)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C33H42N4O6/c1-42-26-11-10-22(18-27(26)43-2)31-24-8-4-5-9-25(24)32(41)37(34-31)23-12-16-35(17-13-23)30(40)21-36-28(38)19-33(20-29(36)39)14-6-3-7-15-33/h4-5,10-11,18,23-25H,3,6-9,12-17,19-21H2,1-2H3/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527531
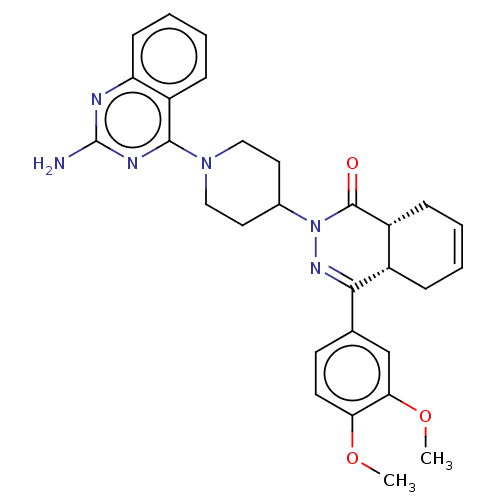
(CHEMBL4524402)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3ccccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C29H32N6O3/c1-37-24-12-11-18(17-25(24)38-2)26-20-7-3-4-8-21(20)28(36)35(33-26)19-13-15-34(16-14-19)27-22-9-5-6-10-23(22)31-29(30)32-27/h3-6,9-12,17,19-21H,7-8,13-16H2,1-2H3,(H2,30,31,32)/t20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50079527

((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 328-36 (2002)
Article DOI: 10.1124/jpet.302.1.328
BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527528
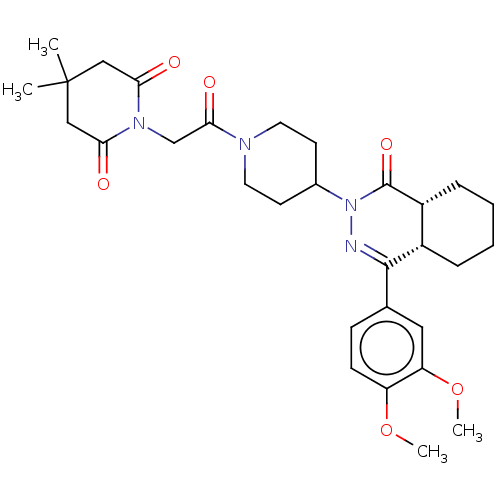
(CHEMBL4441871)Show SMILES [H][C@@]12CCCC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:9| Show InChI InChI=1S/C30H40N4O6/c1-30(2)16-25(35)33(26(36)17-30)18-27(37)32-13-11-20(12-14-32)34-29(38)22-8-6-5-7-21(22)28(31-34)19-9-10-23(39-3)24(15-19)40-4/h9-10,15,20-22H,5-8,11-14,16-18H2,1-4H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527550
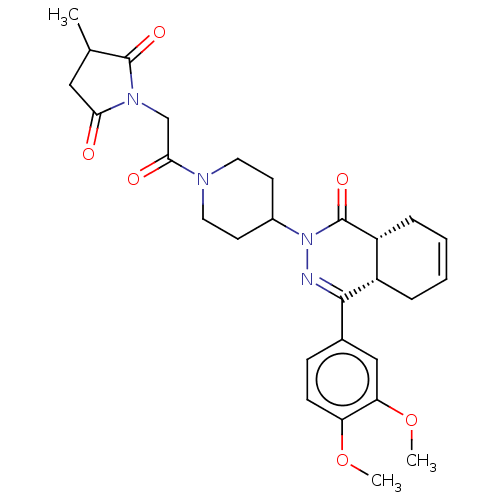
(CHEMBL4461456)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)C1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C28H34N4O6/c1-17-14-24(33)31(27(17)35)16-25(34)30-12-10-19(11-13-30)32-28(36)21-7-5-4-6-20(21)26(29-32)18-8-9-22(37-2)23(15-18)38-3/h4-5,8-9,15,17,19-21H,6-7,10-14,16H2,1-3H3/t17?,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM112780
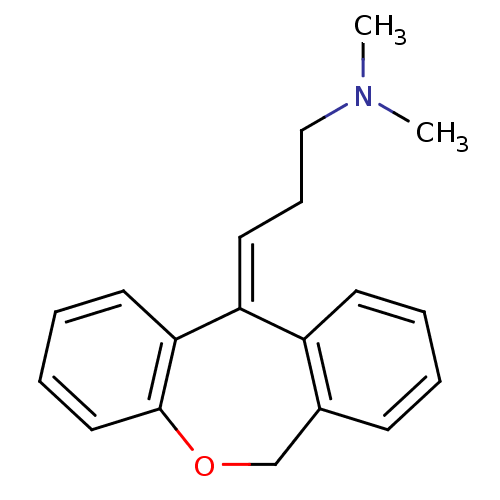
(US8629135, SW-07)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50079527

((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from human histamine H1 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8195-206 (2011)
Article DOI: 10.1021/jm2011589
BindingDB Entry DOI: 10.7270/Q2QF8T85 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50099623
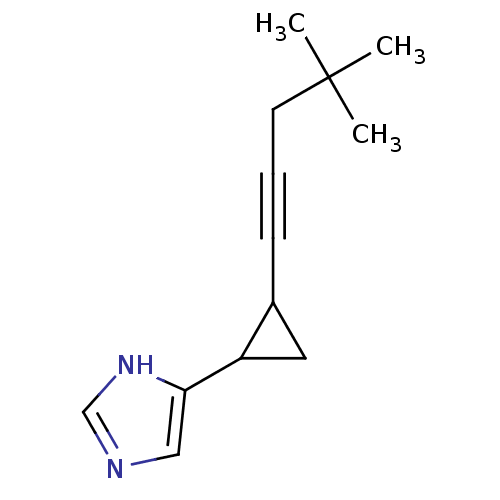
(4-[2-(4,4-Dimethyl-pent-1-ynyl)-cyclopropyl]-1H-im...)Show InChI InChI=1S/C13H18N2/c1-13(2,3)6-4-5-10-7-11(10)12-8-14-9-15-12/h8-11H,6-7H2,1-3H3,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Ability to displace [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527535
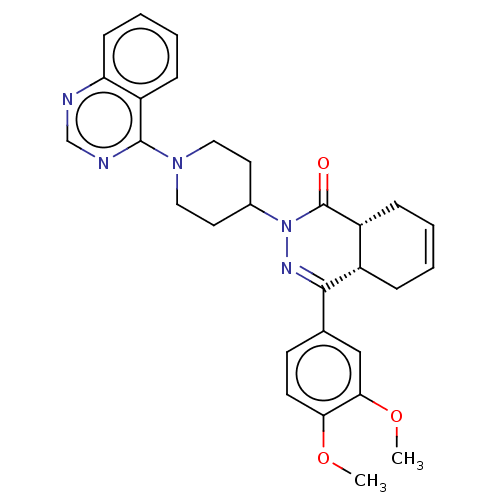
(CHEMBL4589927)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ncnc3ccccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C29H31N5O3/c1-36-25-12-11-19(17-26(25)37-2)27-21-7-3-4-8-22(21)29(35)34(32-27)20-13-15-33(16-14-20)28-23-9-5-6-10-24(23)30-18-31-28/h3-6,9-12,17-18,20-22H,7-8,13-16H2,1-2H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527533
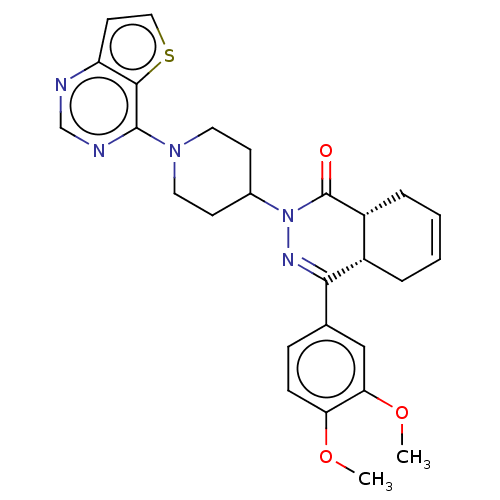
(CHEMBL4592553)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ncnc3ccsc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H29N5O3S/c1-34-22-8-7-17(15-23(22)35-2)24-19-5-3-4-6-20(19)27(33)32(30-24)18-9-12-31(13-10-18)26-25-21(11-14-36-25)28-16-29-26/h3-4,7-8,11,14-16,18-20H,5-6,9-10,12-13H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50079527

((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from human wild type N-terminal hemagglutinin-tagged histamine H1 receptor expressed in HEK293T cells after 4 hrs by m... |
J Med Chem 59: 9047-9061 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00981
BindingDB Entry DOI: 10.7270/Q2G44S8Z |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50079527

((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 328-36 (2002)
Article DOI: 10.1124/jpet.302.1.328
BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527555
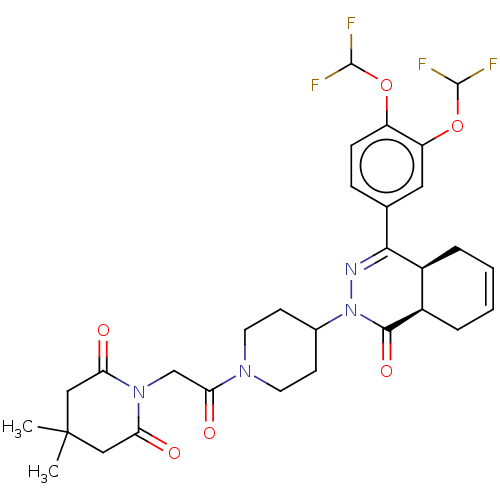
(CHEMBL4530777)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC(F)F)c(OC(F)F)c1 |r,c:3,9| Show InChI InChI=1S/C30H34F4N4O6/c1-30(2)14-23(39)37(24(40)15-30)16-25(41)36-11-9-18(10-12-36)38-27(42)20-6-4-3-5-19(20)26(35-38)17-7-8-21(43-28(31)32)22(13-17)44-29(33)34/h3-4,7-8,13,18-20,28-29H,5-6,9-12,14-16H2,1-2H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527560
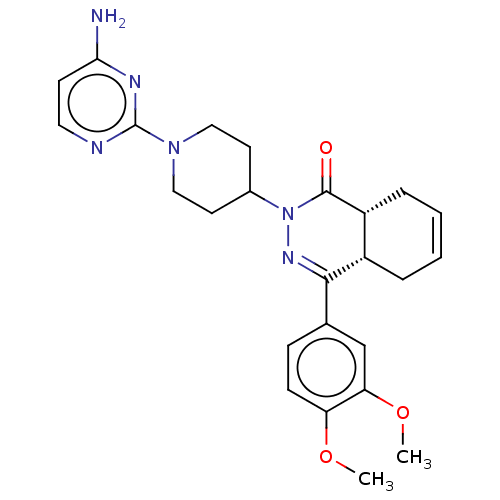
(CHEMBL4444578)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nccc(N)n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H30N6O3/c1-33-20-8-7-16(15-21(20)34-2)23-18-5-3-4-6-19(18)24(32)31(29-23)17-10-13-30(14-11-17)25-27-12-9-22(26)28-25/h3-4,7-9,12,15,17-19H,5-6,10-11,13-14H2,1-2H3,(H2,26,27,28)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527567
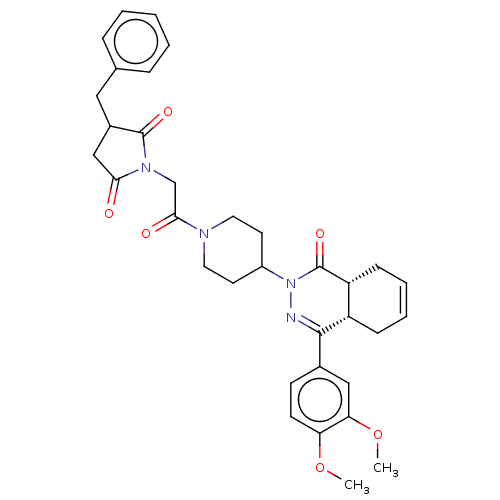
(CHEMBL4473426)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)C(=O)CN1C(=O)CC(Cc3ccccc3)C1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C34H38N4O6/c1-43-28-13-12-23(19-29(28)44-2)32-26-10-6-7-11-27(26)34(42)38(35-32)25-14-16-36(17-15-25)31(40)21-37-30(39)20-24(33(37)41)18-22-8-4-3-5-9-22/h3-9,12-13,19,24-27H,10-11,14-18,20-21H2,1-2H3/t24?,26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50079527

((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 328-36 (2002)
Article DOI: 10.1124/jpet.302.1.328
BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.339 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam
| Assay Description
Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... |
J Med Chem 51: 2944-53 (2008)
Article DOI: 10.1021/jm7014149
BindingDB Entry DOI: 10.7270/Q24F1P2W |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527554
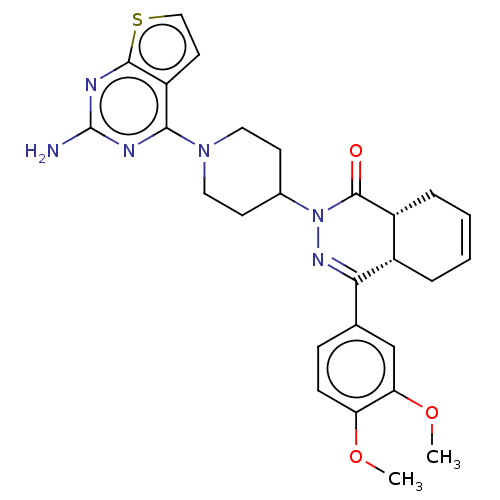
(CHEMBL4460623)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3sccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H30N6O3S/c1-35-21-8-7-16(15-22(21)36-2)23-18-5-3-4-6-19(18)26(34)33(31-23)17-9-12-32(13-10-17)24-20-11-14-37-25(20)30-27(28)29-24/h3-4,7-8,11,14-15,17-19H,5-6,9-10,12-13H2,1-2H3,(H2,28,29,30)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527559
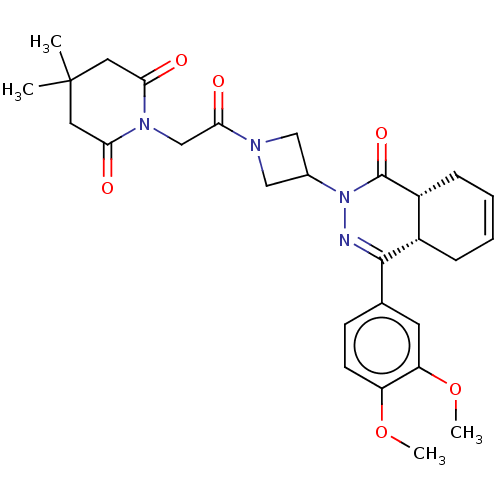
(CHEMBL4591859)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)C(=O)CN1C(=O)CC(C)(C)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C28H34N4O6/c1-28(2)12-23(33)31(24(34)13-28)16-25(35)30-14-18(15-30)32-27(36)20-8-6-5-7-19(20)26(29-32)17-9-10-21(37-3)22(11-17)38-4/h5-6,9-11,18-20H,7-8,12-16H2,1-4H3/t19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay |
J Med Chem 61: 3870-3888 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01670
BindingDB Entry DOI: 10.7270/Q2P84FF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50031228
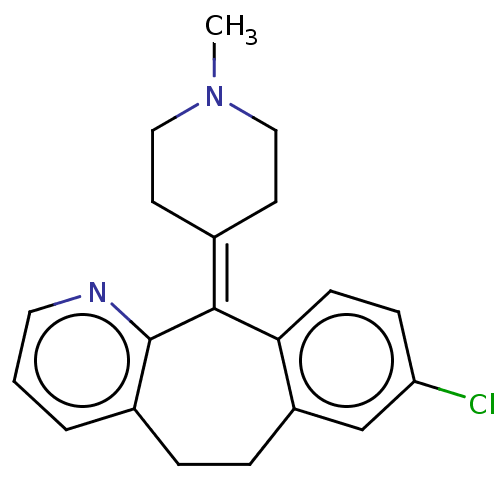
(CHEMBL420316)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C20H21ClN2/c1-23-11-8-14(9-12-23)19-18-7-6-17(21)13-16(18)5-4-15-3-2-10-22-20(15)19/h2-3,6-7,10,13H,4-5,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... |
J Med Chem 62: 6630-6644 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00447
BindingDB Entry DOI: 10.7270/Q2HH6PFT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50099622
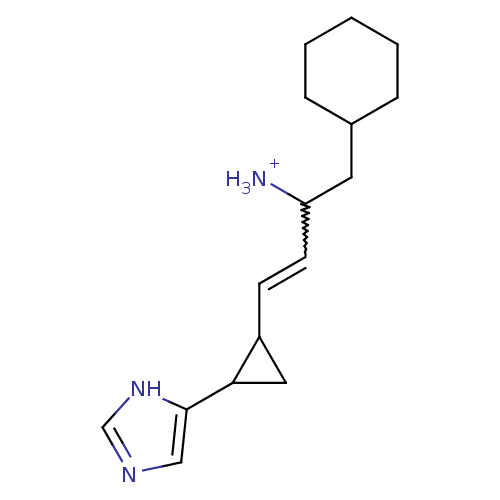
(1-Cyclohexylmethyl-3-[2-(1H-imidazol-4-yl)-cyclopr...)Show SMILES [NH3+]C(CC1CCCCC1)C=CC1CC1c1cnc[nH]1 |w:9.9| Show InChI InChI=1S/C16H25N3/c17-14(8-12-4-2-1-3-5-12)7-6-13-9-15(13)16-10-18-11-19-16/h6-7,10-15H,1-5,8-9,17H2,(H,18,19)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Ability to displace [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.407 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam
| Assay Description
Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... |
J Med Chem 51: 2944-53 (2008)
Article DOI: 10.1021/jm7014149
BindingDB Entry DOI: 10.7270/Q24F1P2W |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50516710
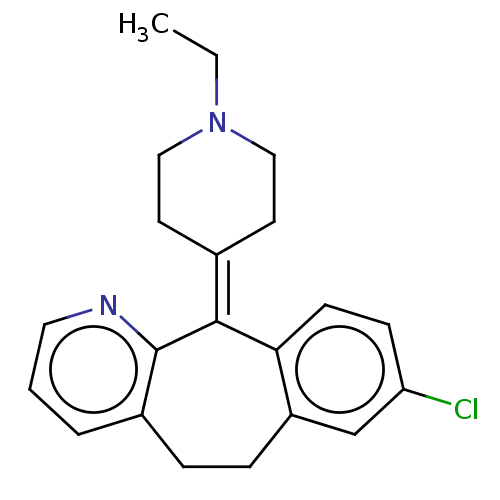
(CHEMBL4469024)Show SMILES [#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C21H23ClN2/c1-2-24-12-9-15(10-13-24)20-19-8-7-18(22)14-17(19)6-5-16-4-3-11-23-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... |
J Med Chem 62: 6630-6644 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00447
BindingDB Entry DOI: 10.7270/Q2HH6PFT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50053631
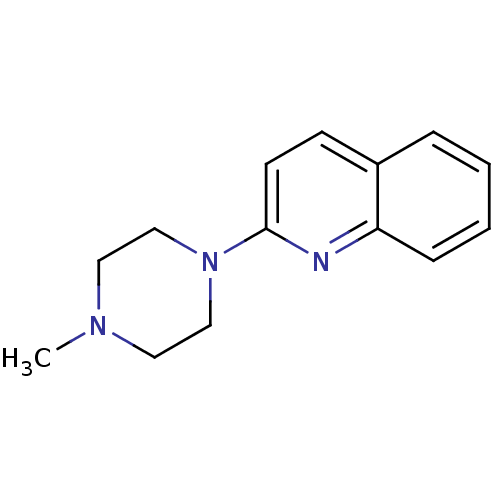
(2-(4-Methyl-piperazin-1-yl)-quinoline | 2-(4-methy...)Show InChI InChI=1S/C14H17N3/c1-16-8-10-17(11-9-16)14-7-6-12-4-2-3-5-13(12)15-14/h2-7H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from 5HT3A receptor expressed in HEK293 cells after 24 hrs by scintillation counting in presence of quipazine |
J Med Chem 55: 8603-14 (2012)
Article DOI: 10.1021/jm300801u
BindingDB Entry DOI: 10.7270/Q2RF5W5T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50150945
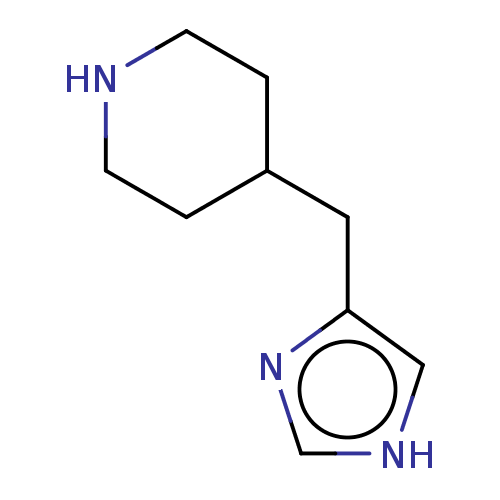
(CHEBI:81390 | Immepip)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by ChEMBL
| Assay Description
Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor |
J Med Chem 46: 5445-57 (2003)
Article DOI: 10.1021/jm030905y
BindingDB Entry DOI: 10.7270/Q2FN18X0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8136-47 (2011)
Article DOI: 10.1021/jm201042n
BindingDB Entry DOI: 10.7270/Q2MW2JDX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50150945

(CHEBI:81390 | Immepip)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-R-methylhistamine binding to SK-N-MC cell membranes expressing human H3 receptor |
J Med Chem 48: 2100-7 (2005)
Article DOI: 10.1021/jm049475h
BindingDB Entry DOI: 10.7270/Q28K7CVC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527525
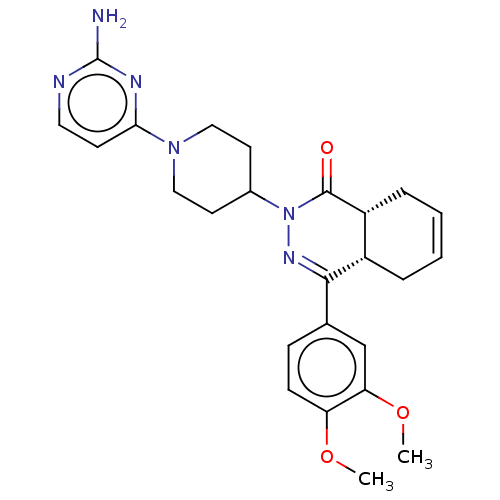
(CHEMBL4522249)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1ccnc(N)n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H30N6O3/c1-33-20-8-7-16(15-21(20)34-2)23-18-5-3-4-6-19(18)24(32)31(29-23)17-10-13-30(14-11-17)22-9-12-27-25(26)28-22/h3-4,7-9,12,15,17-19H,5-6,10-11,13-14H2,1-2H3,(H2,26,27,28)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527529
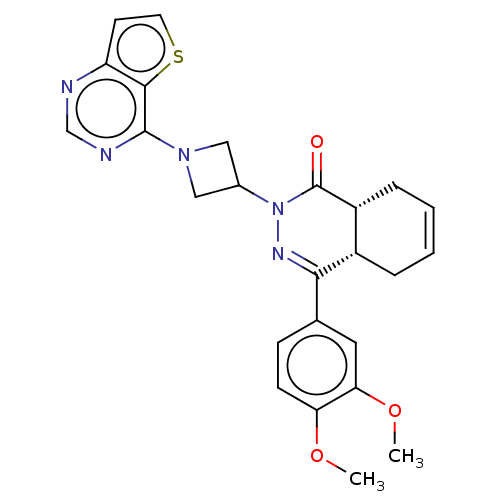
(CHEMBL4454600)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)c1ncnc3ccsc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C25H25N5O3S/c1-32-20-8-7-15(11-21(20)33-2)22-17-5-3-4-6-18(17)25(31)30(28-22)16-12-29(13-16)24-23-19(9-10-34-23)26-14-27-24/h3-4,7-11,14,16-18H,5-6,12-13H2,1-2H3/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 328-36 (2002)
Article DOI: 10.1124/jpet.302.1.328
BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM86032

(trans-H2-PAT(-) | trans-PAT)Show InChI InChI=1S/C18H21N/c1-19(2)16-12-15-10-6-7-11-17(15)18(13-16)14-8-4-3-5-9-14/h3-11,16,18H,12-13H2,1-2H3/t16-,18-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 328-36 (2002)
Article DOI: 10.1124/jpet.302.1.328
BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 90: 8547-51 (1993)
Article DOI: 10.1073/pnas.90.18.8547
BindingDB Entry DOI: 10.7270/Q29Z93D3 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50335557

(CHEMBL1652605 | N,N-dipropyl-N'-[4-({[(1H-imidazol...)Show SMILES CCCN(CCC)CCCCN(C)Cc1ccc(CN(Cc2ncc[nH]2)Cc2nccn2C)cc1 Show InChI InChI=1S/C28H45N7/c1-5-16-34(17-6-2)19-8-7-18-32(3)21-25-9-11-26(12-10-25)22-35(23-27-29-13-14-30-27)24-28-31-15-20-33(28)4/h9-15,20H,5-8,16-19,21-24H2,1-4H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22916
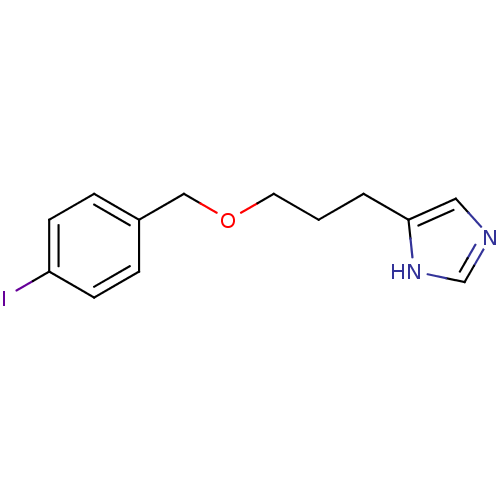
(5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...)Show InChI InChI=1S/C13H15IN2O/c14-12-5-3-11(4-6-12)9-17-7-1-2-13-8-15-10-16-13/h3-6,8,10H,1-2,7,9H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50516713
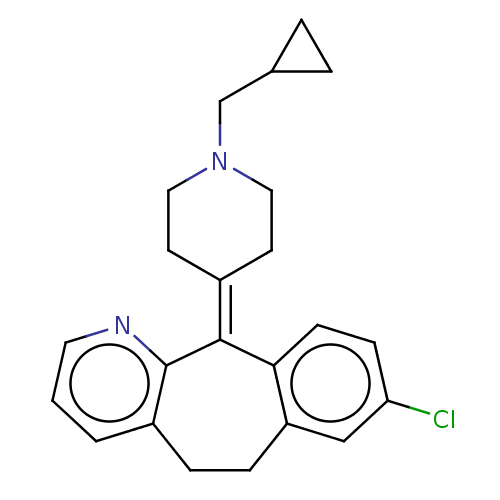
(CHEMBL4448535)Show SMILES Clc1ccc2c(-[#6]-[#6]-c3cccnc3\[#6]-2=[#6]-2/[#6]-[#6]-[#7](-[#6]-[#6]-3-[#6]-[#6]-3)-[#6]-[#6]-2)c1 Show InChI InChI=1S/C23H25ClN2/c24-20-7-8-21-19(14-20)6-5-18-2-1-11-25-23(18)22(21)17-9-12-26(13-10-17)15-16-3-4-16/h1-2,7-8,11,14,16H,3-6,9-10,12-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]mepyramine from N-terminal HA-tagged H1R (unknown origin) expressed in HEK293T cells measured after 4 hrs by microbeta scintillat... |
J Med Chem 62: 6630-6644 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00447
BindingDB Entry DOI: 10.7270/Q2HH6PFT |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50427452
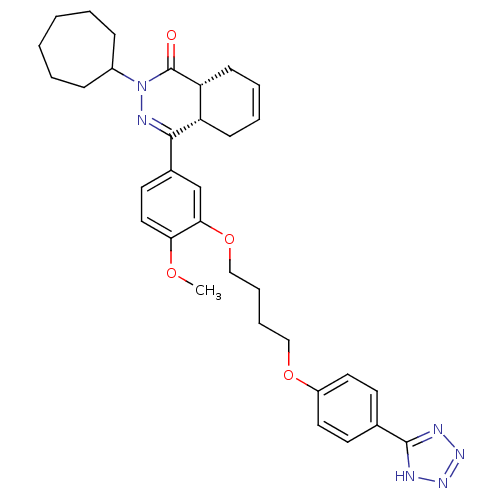
(CHEMBL2326941)Show SMILES COc1ccc(cc1OCCCCOc1ccc(cc1)-c1nnn[nH]1)C1=NN(C2CCCCCC2)C(=O)[C@@H]2CC=CC[C@H]12 |r,c:43,t:28| Show InChI InChI=1S/C33H40N6O4/c1-41-29-19-16-24(31-27-12-6-7-13-28(27)33(40)39(36-31)25-10-4-2-3-5-11-25)22-30(29)43-21-9-8-20-42-26-17-14-23(15-18-26)32-34-37-38-35-32/h6-7,14-19,22,25,27-28H,2-5,8-13,20-21H2,1H3,(H,34,35,37,38)/t27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay |
J Med Chem 61: 3870-3888 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01670
BindingDB Entry DOI: 10.7270/Q2P84FF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22916

(5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...)Show InChI InChI=1S/C13H15IN2O/c14-12-5-3-11(4-6-12)9-17-7-1-2-13-8-15-10-16-13/h3-6,8,10H,1-2,7,9H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8136-47 (2011)
Article DOI: 10.1021/jm201042n
BindingDB Entry DOI: 10.7270/Q2MW2JDX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527527
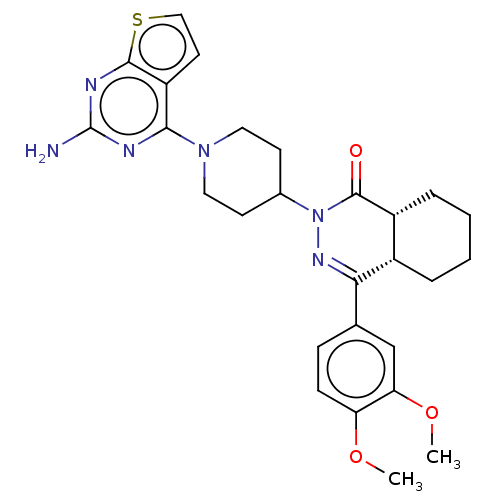
(CHEMBL4466777)Show SMILES [H][C@@]12CCCC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)nc3sccc13)C2=O)c1ccc(OC)c(OC)c1 |r,c:9| Show InChI InChI=1S/C27H32N6O3S/c1-35-21-8-7-16(15-22(21)36-2)23-18-5-3-4-6-19(18)26(34)33(31-23)17-9-12-32(13-10-17)24-20-11-14-37-25(20)30-27(28)29-24/h7-8,11,14-15,17-19H,3-6,9-10,12-13H2,1-2H3,(H2,28,29,30)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527530
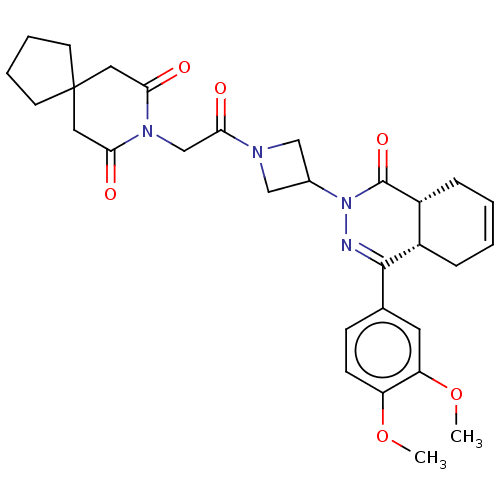
(CHEMBL4461705)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CN(C1)C(=O)CN1C(=O)CC3(CCCC3)CC1=O)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C30H36N4O6/c1-39-23-10-9-19(13-24(23)40-2)28-21-7-3-4-8-22(21)29(38)34(31-28)20-16-32(17-20)27(37)18-33-25(35)14-30(15-26(33)36)11-5-6-12-30/h3-4,9-10,13,20-22H,5-8,11-12,14-18H2,1-2H3/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50527534
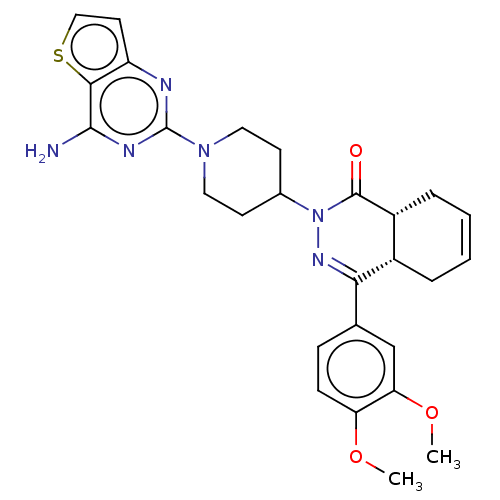
(CHEMBL4472307)Show SMILES [H][C@@]12CC=CC[C@]1([H])C(=NN(C1CCN(CC1)c1nc(N)c3sccc3n1)C2=O)c1ccc(OC)c(OC)c1 |r,c:3,9| Show InChI InChI=1S/C27H30N6O3S/c1-35-21-8-7-16(15-22(21)36-2)23-18-5-3-4-6-19(18)26(34)33(31-23)17-9-12-32(13-10-17)27-29-20-11-14-37-24(20)25(28)30-27/h3-4,7-8,11,14-15,17-19H,5-6,9-10,12-13H2,1-2H3,(H2,28,29,30)/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based |
J Med Chem 63: 3485-3507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00985
BindingDB Entry DOI: 10.7270/Q20005JV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM35938
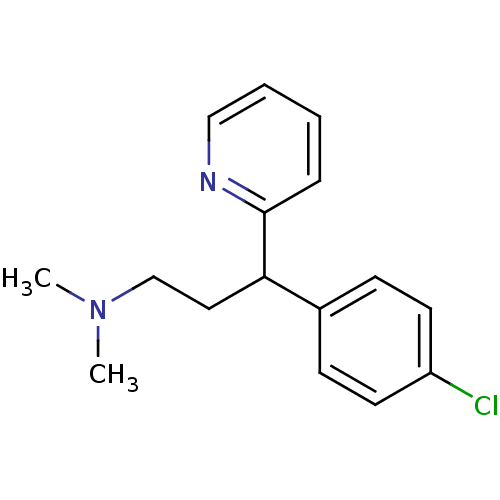
(1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...)Show InChI InChI=1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 328-36 (2002)
Article DOI: 10.1124/jpet.302.1.328
BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50292411
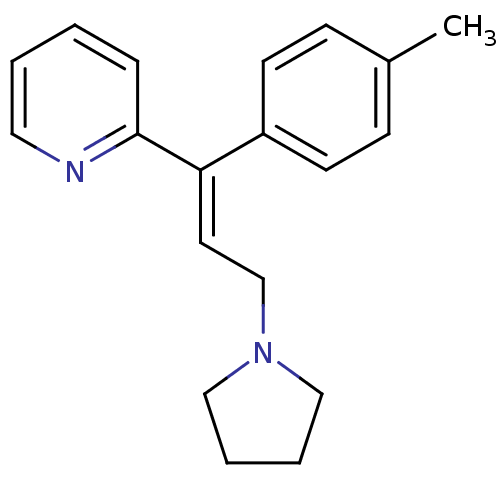
((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...)Show InChI InChI=1S/C19H22N2/c1-16-7-9-17(10-8-16)18(19-6-2-3-12-20-19)11-15-21-13-4-5-14-21/h2-3,6-12H,4-5,13-15H2,1H3/b18-11+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 328-36 (2002)
Article DOI: 10.1124/jpet.302.1.328
BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 328-36 (2002)
Article DOI: 10.1124/jpet.302.1.328
BindingDB Entry DOI: 10.7270/Q2JQ0ZK1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data