| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor alpha |
|---|
| Ligand | BDBM50521960 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1885855 (CHEMBL4387437) |
|---|
| EC50 | >4010±n/a nM |
|---|
| Citation |  Miyachi, H; Yuzuriha, T; Tabata, R; Fukuda, S; Nunomura, K; Lin, B; Kobayashi, T; Ishimoto, K; Doi, T; Tachibana, K Structural development of 1H-pyrazolo-[3,4-b]pyridine-4-carboxylic acid derivatives as human peroxisome proliferator-activated receptor alpha (PPAR?)-selective agonists. Bioorg Med Chem Lett29:2124-2128 (2019) [PubMed] Article Miyachi, H; Yuzuriha, T; Tabata, R; Fukuda, S; Nunomura, K; Lin, B; Kobayashi, T; Ishimoto, K; Doi, T; Tachibana, K Structural development of 1H-pyrazolo-[3,4-b]pyridine-4-carboxylic acid derivatives as human peroxisome proliferator-activated receptor alpha (PPAR?)-selective agonists. Bioorg Med Chem Lett29:2124-2128 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor alpha |
|---|
| Name: | Peroxisome proliferator-activated receptor alpha |
|---|
| Synonyms: | NR1C1 | Nuclear receptor subfamily 1 group C member 1 | PPAR | PPAR alpha/gamma | PPAR-alpha | PPARA | PPARA_HUMAN | Peroxisome Proliferator-Activated Receptor alpha | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor alpha (PPAR alpha) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 52222.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q07869 |
|---|
| Residue: | 468 |
|---|
| Sequence: | MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSC
PGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACE
GCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSE
KAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFV
IHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANL
DLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFD
FAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDI
FLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY
|
|
|
|---|
| BDBM50521960 |
|---|
| n/a |
|---|
| Name | BDBM50521960 |
|---|
| Synonyms: | CHEMBL4538761 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H16BrN3O2 |
|---|
| Mol. Mass. | 374.232 |
|---|
| SMILES | CC(C)c1nn(-c2ccc(Br)cc2)c2nc(C)cc(C(O)=O)c12 |
|---|
| Structure | 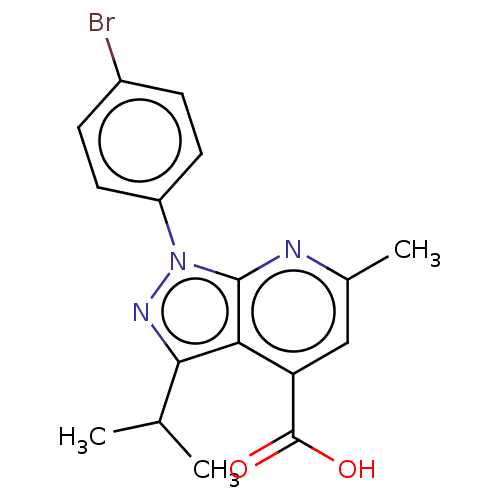
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Miyachi, H; Yuzuriha, T; Tabata, R; Fukuda, S; Nunomura, K; Lin, B; Kobayashi, T; Ishimoto, K; Doi, T; Tachibana, K Structural development of 1H-pyrazolo-[3,4-b]pyridine-4-carboxylic acid derivatives as human peroxisome proliferator-activated receptor alpha (PPAR?)-selective agonists. Bioorg Med Chem Lett29:2124-2128 (2019) [PubMed] Article
Miyachi, H; Yuzuriha, T; Tabata, R; Fukuda, S; Nunomura, K; Lin, B; Kobayashi, T; Ishimoto, K; Doi, T; Tachibana, K Structural development of 1H-pyrazolo-[3,4-b]pyridine-4-carboxylic acid derivatives as human peroxisome proliferator-activated receptor alpha (PPAR?)-selective agonists. Bioorg Med Chem Lett29:2124-2128 (2019) [PubMed] Article