| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Folylpolyglutamate synthase, mitochondrial |
|---|
| Ligand | BDBM50116251 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_68690 |
|---|
| IC50 | 8600±n/a nM |
|---|
| Citation |  Tsukamoto, T; Flanary, JM; Rojas, C; Slusher, BS; Valiaeva, N; Coward, JK Phosphonate and phosphinate analogues of N-acylated gamma-glutamylglutamate. potent inhibitors of glutamate carboxypeptidase II. Bioorg Med Chem Lett12:2189-92 (2002) [PubMed] Tsukamoto, T; Flanary, JM; Rojas, C; Slusher, BS; Valiaeva, N; Coward, JK Phosphonate and phosphinate analogues of N-acylated gamma-glutamylglutamate. potent inhibitors of glutamate carboxypeptidase II. Bioorg Med Chem Lett12:2189-92 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Folylpolyglutamate synthase, mitochondrial |
|---|
| Name: | Folylpolyglutamate synthase, mitochondrial |
|---|
| Synonyms: | FOLC_HUMAN | FPGS | Folylpoly-gamma-glutamate synthetase | Folylpolyglutamate synthase, mitochondrial | Tetrahydrofolate synthase | Tetrahydrofolylpolyglutamate synthase |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 64621.95 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_68676 |
|---|
| Residue: | 587 |
|---|
| Sequence: | MSRARSHLRAALFLAAASARGITTQVAARRGLSAWPVPQEPSMEYQDAVRMLNTLQTNAG
YLEQVKRQRGDPQTQLEAMELYLARSGLQVEDLDRLNIIHVTGTKGKGSTCAFTECILRS
YGLKTGFFSSPHLVQVRERIRINGQPISPELFTKYFWRLYHRLEETKDGSCVSMPPYFRF
LTLMAFHVFLQEKVDLAVVEVGIGGAYDCTNIIRKPVVCGVSSLGIDHTSLLGDTVEKIA
WQKGGIFKQGVPAFTVLQPEGPLAVLRDRAQQISCPLYLCPMLEALEEGGPPLTLGLEGE
HQRSNAALALQLAHCWLQRQDRHGAGEPKASRPGLLWQLPLAPVFQPTSHMRLGLRNTEW
PGRTQVLRRGPLTWYLDGAHTASSAQACVRWFRQALQGRERPSGGPEVRVLLFNATGDRD
PAALLKLLQPCQFDYAVFCPNLTEVSSTGNADQQNFTVTLDQVLLRCLEHQQHWNHLDEE
QASPDLWSAPSPEPGGSASLLLAPHPPHTCSASSLVFSCISHALQWISQGRDPIFQPPSP
PKGLLTHPVAHSGASILREAAAIHVLVTGSLHLVGGVLKLLEPALSQ
|
|
|
|---|
| BDBM50116251 |
|---|
| n/a |
|---|
| Name | BDBM50116251 |
|---|
| Synonyms: | 2-{[3-(4-Amino-benzoylamino)-3-carboxy-propyl]-hydroxy-phosphinoyloxy}-pentanedioic acid | CHEMBL307085 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H21N2O10P |
|---|
| Mol. Mass. | 432.3191 |
|---|
| SMILES | Nc1ccc(cc1)C(=O)N[C@@H](CCP(O)(=O)O[C@@H](CCC(O)=O)C(O)=O)C(O)=O |
|---|
| Structure | 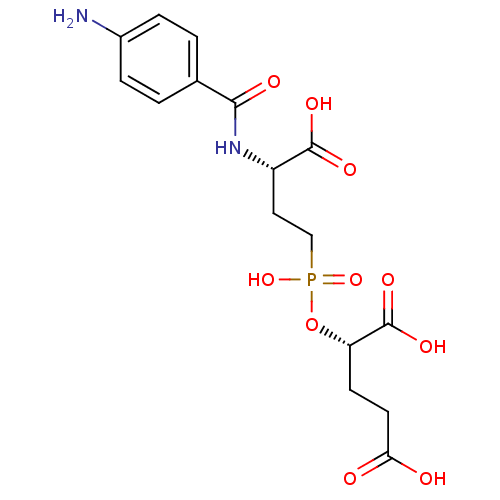
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tsukamoto, T; Flanary, JM; Rojas, C; Slusher, BS; Valiaeva, N; Coward, JK Phosphonate and phosphinate analogues of N-acylated gamma-glutamylglutamate. potent inhibitors of glutamate carboxypeptidase II. Bioorg Med Chem Lett12:2189-92 (2002) [PubMed]
Tsukamoto, T; Flanary, JM; Rojas, C; Slusher, BS; Valiaeva, N; Coward, JK Phosphonate and phosphinate analogues of N-acylated gamma-glutamylglutamate. potent inhibitors of glutamate carboxypeptidase II. Bioorg Med Chem Lett12:2189-92 (2002) [PubMed]