

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM50129200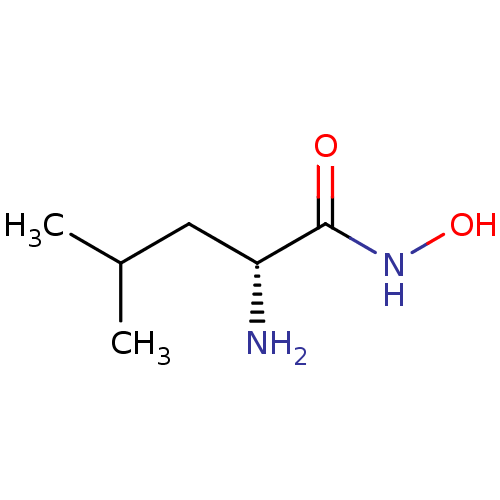 ((R)-2-Amino-4-methyl-pentanoic acid hydroxyamide |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of metalloprotease from family M28, Aeromonas proteolytica aminopeptidase | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM50129202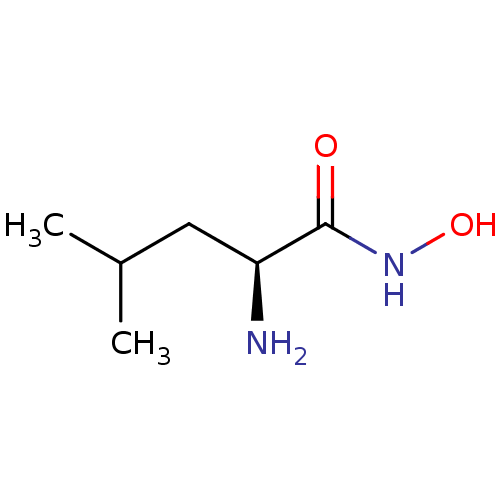 ((S)-2-Amino-4-methyl-pentanoic acid hydroxyamide |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of metalloprotease from family M28, Aeromonas proteolytica aminopeptidase | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116251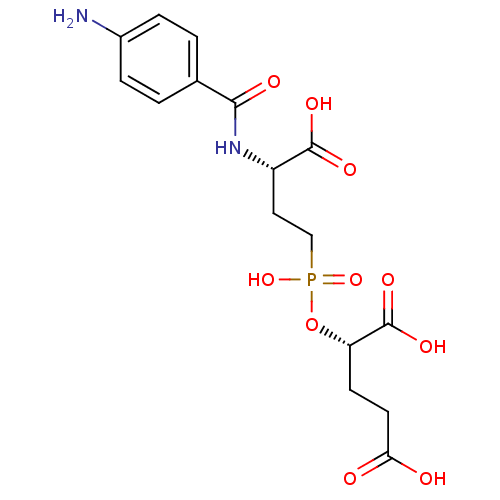 (2-{[3-(4-Amino-benzoylamino)-3-carboxy-propyl]-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116253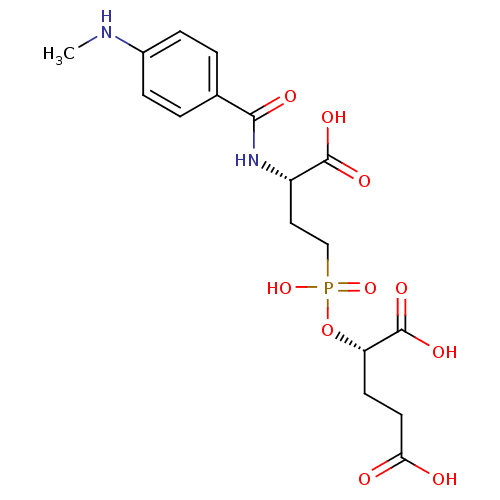 (2-{[3-Carboxy-3-(4-methylamino-benzoylamino)-propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116250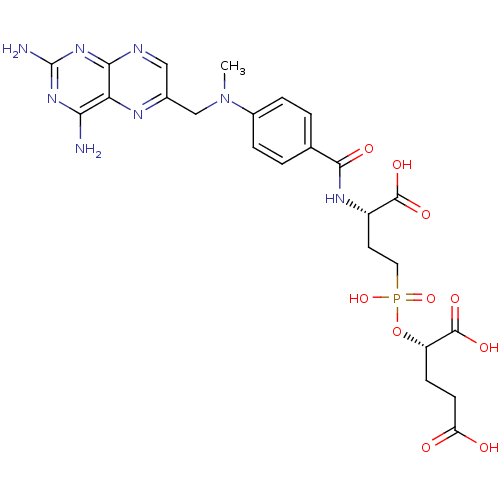 (2-[(3-Carboxy-3-{4-[(2,4-diamino-pteridin-6-ylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116252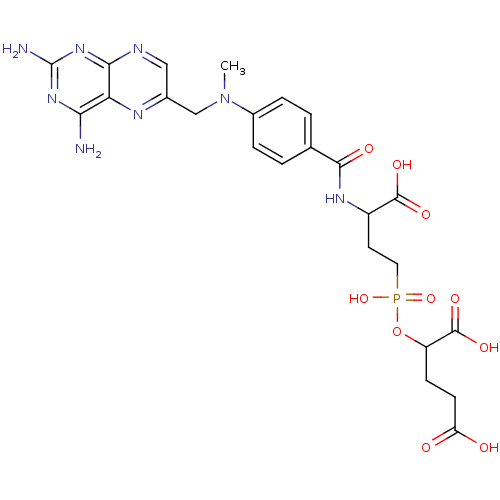 (2-[(3-Carboxy-3-{4-[(2,4-diamino-pteridin-6-ylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50116252 (2-[(3-Carboxy-3-{4-[(2,4-diamino-pteridin-6-ylmeth...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Folylpoly-gamma-glutamyl synthetase | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17752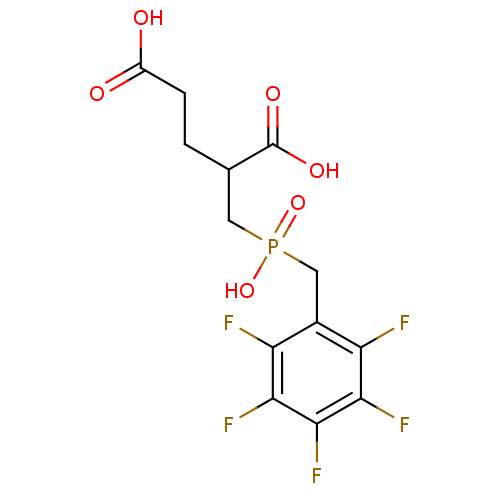 (2-({hydroxy[(2,3,4,5,6-pentafluorophenyl)methyl]ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17755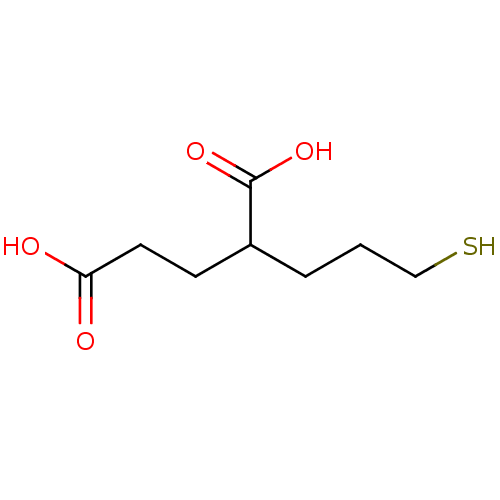 (2-(3-sulfanylpropyl)pentanedioic acid | 2-MPPA | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50116250 (2-[(3-Carboxy-3-{4-[(2,4-diamino-pteridin-6-ylmeth...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Folylpoly-gamma-glutamyl synthetase | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17779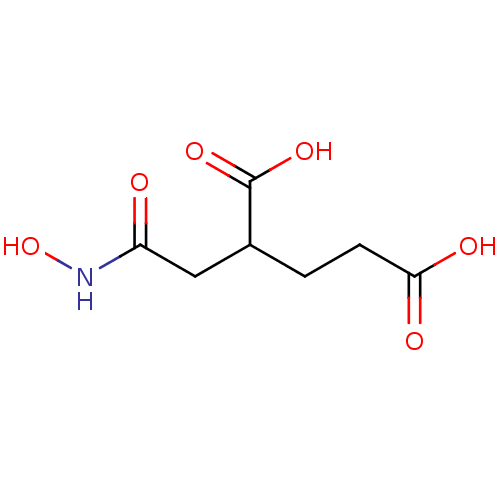 (2-[(hydroxycarbamoyl)methyl]pentanedioic acid | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50129199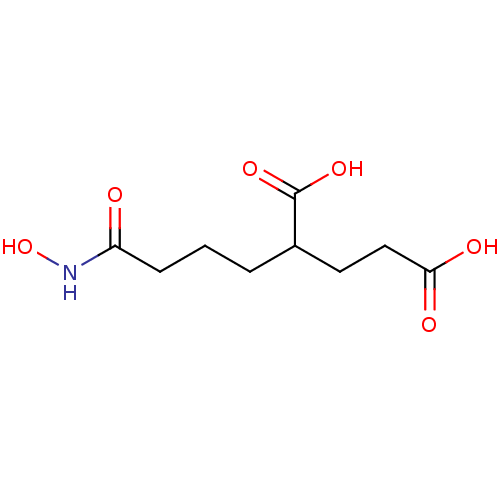 (2-(3-Hydroxycarbamoyl-propyl)-pentanedioic acid | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50129195 (2-(2-Hydroxycarbamoyl-ethyl)-pentanedioic acid | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50129193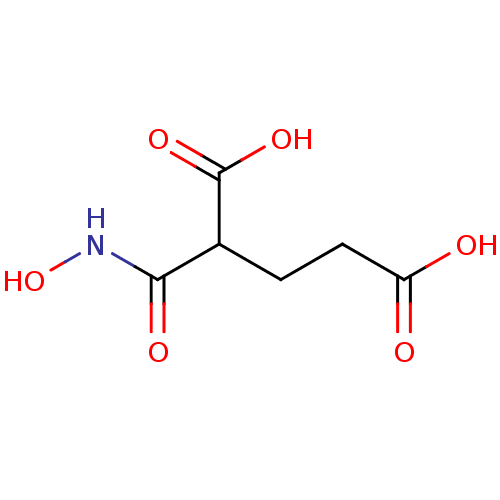 (2-Hydroxycarbamoyl-pentanedioic acid | CHEMBL43272...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50116253 (2-{[3-Carboxy-3-(4-methylamino-benzoylamino)-propy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Folylpoly-gamma-glutamyl synthetase | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50116251 (2-{[3-(4-Amino-benzoylamino)-3-carboxy-propyl]-hyd...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Folylpoly-gamma-glutamyl synthetase | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17781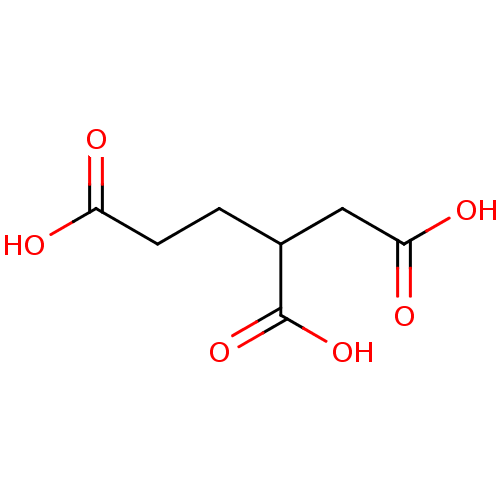 (CHEMBL65860 | butane-1,2,4-tricarboxylic acid | ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50129197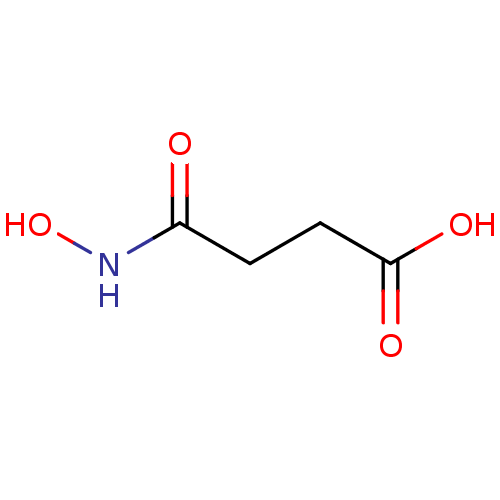 (4-(Hydroxyamino)-4-oxobutanoic acid | CHEMBL51979 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50129196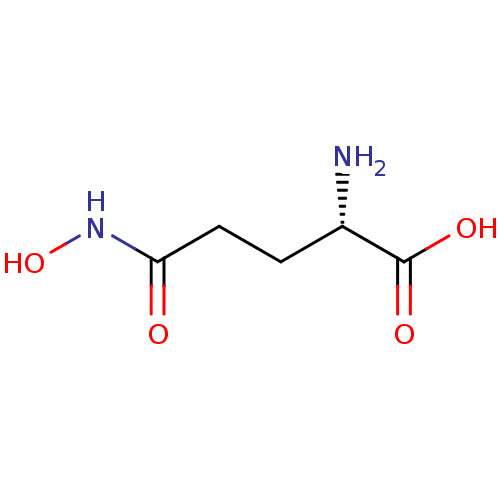 ((S)-2-Amino-4-hydroxycarbamoyl-butyric acid | (S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50129203 ((R)-3-Acetylamino-N-hydroxy-succinamic acid | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50129194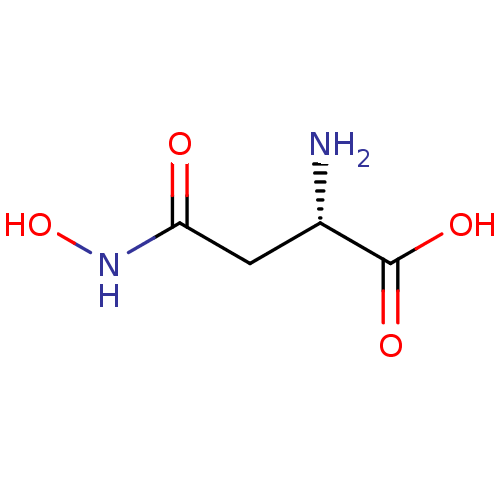 ((2S)-2-amino-4-(hydroxyamino)-4-oxobutanoic acidN(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50129201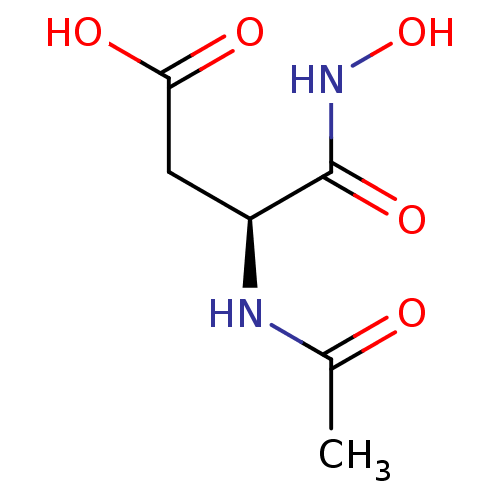 ((S)-3-Acetylamino-N-hydroxy-succinamic acid | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50129198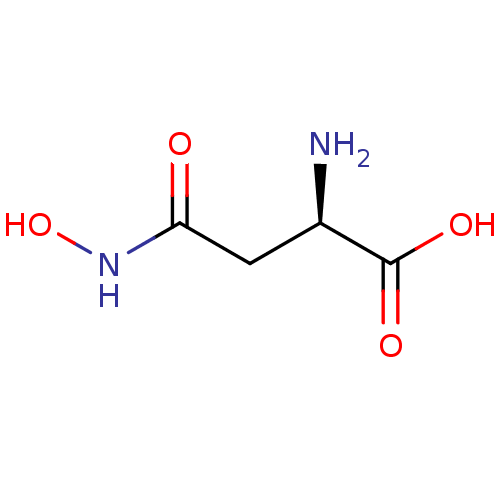 ((R)-2-Amino-N-hydroxy-succinamic acid | CHEMBL4139...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17755 (2-(3-sulfanylpropyl)pentanedioic acid | 2-MPPA | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17779 (2-[(hydroxycarbamoyl)methyl]pentanedioic acid | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17752 (2-({hydroxy[(2,3,4,5,6-pentafluorophenyl)methyl]ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration required against Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50129199 (2-(3-Hydroxycarbamoyl-propyl)-pentanedioic acid | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||