| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50121357 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_195204 (CHEMBL798996) |
|---|
| IC50 | 4120±n/a nM |
|---|
| Citation |  Barreca, ML; Balzarini, J; Chimirri, A; De Clercq, E; De Luca, L; Höltje, HD; Höltje, M; Monforte, AM; Monforte, P; Pannecouque, C; Rao, A; Zappalà, M Design, synthesis, structure-activity relationships, and molecular modeling studies of 2,3-diaryl-1,3-thiazolidin-4-ones as potent anti-HIV agents. J Med Chem45:5410-3 (2002) [PubMed] Barreca, ML; Balzarini, J; Chimirri, A; De Clercq, E; De Luca, L; Höltje, HD; Höltje, M; Monforte, AM; Monforte, P; Pannecouque, C; Rao, A; Zappalà, M Design, synthesis, structure-activity relationships, and molecular modeling studies of 2,3-diaryl-1,3-thiazolidin-4-ones as potent anti-HIV agents. J Med Chem45:5410-3 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50121357 |
|---|
| n/a |
|---|
| Name | BDBM50121357 |
|---|
| Synonyms: | 2-(2,6-Difluoro-phenyl)-3-(4,6-dimethyl-pyridin-2-yl)-thiazolidin-4-one | 2-(2,6-Difluorophenyl)-3-(4,6-dimethylpyridin-2-yl)-l,3-thiazolidin-4-one | 2-(2,6-difluorophenyl)-3-(4,6-dimethylpyridin-2-yl)thiazolidin-4-one | CHEMBL150025 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H14F2N2OS |
|---|
| Mol. Mass. | 320.357 |
|---|
| SMILES | Cc1cc(C)nc(c1)N1C(SCC1=O)c1c(F)cccc1F |
|---|
| Structure | 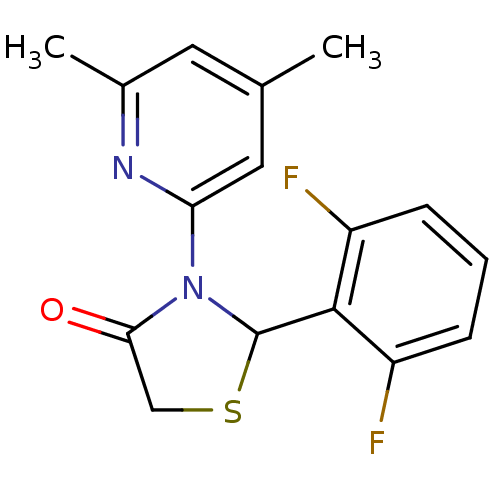
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Barreca, ML; Balzarini, J; Chimirri, A; De Clercq, E; De Luca, L; Höltje, HD; Höltje, M; Monforte, AM; Monforte, P; Pannecouque, C; Rao, A; Zappalà, M Design, synthesis, structure-activity relationships, and molecular modeling studies of 2,3-diaryl-1,3-thiazolidin-4-ones as potent anti-HIV agents. J Med Chem45:5410-3 (2002) [PubMed]
Barreca, ML; Balzarini, J; Chimirri, A; De Clercq, E; De Luca, L; Höltje, HD; Höltje, M; Monforte, AM; Monforte, P; Pannecouque, C; Rao, A; Zappalà, M Design, synthesis, structure-activity relationships, and molecular modeling studies of 2,3-diaryl-1,3-thiazolidin-4-ones as potent anti-HIV agents. J Med Chem45:5410-3 (2002) [PubMed]