| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histamine H3 receptor |
|---|
| Ligand | BDBM50139346 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_83645 |
|---|
| Ki | 8.6±n/a nM |
|---|
| Citation |  Gfesser, GA; Zhang, H; Dinges, J; Fox, GB; Pan, JB; Esbenshade, TA; Yao, BB; Witte, D; Miller, TR; Kang, CH; Krueger, KM; Bennani, YL; Hancock, AA; Faghih, R Structure-activity relationships of non-imidazole H(3) receptor ligands. Part 3: 5-Substituted 3-phenyl-1,2,4-oxadiazoles as potent antagonists. Bioorg Med Chem Lett14:673-6 (2004) [PubMed] Gfesser, GA; Zhang, H; Dinges, J; Fox, GB; Pan, JB; Esbenshade, TA; Yao, BB; Witte, D; Miller, TR; Kang, CH; Krueger, KM; Bennani, YL; Hancock, AA; Faghih, R Structure-activity relationships of non-imidazole H(3) receptor ligands. Part 3: 5-Substituted 3-phenyl-1,2,4-oxadiazoles as potent antagonists. Bioorg Med Chem Lett14:673-6 (2004) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histamine H3 receptor |
|---|
| Name: | Histamine H3 receptor |
|---|
| Synonyms: | G-protein coupled receptor 97 | GPCR97 | HH3R | HISTAMINE H3 | HRH3 | HRH3_HUMAN | Histamine H3 receptor (H3) | Histamine H3L | Histamine receptor (H3 and H4) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 48691.47 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Binding assays were using CHO cells stably expressing hH3R receptors. |
|---|
| Residue: | 445 |
|---|
| Sequence: | MERAPPDGPLNASGALAGEAAAAGGARGFSAAWTAVLAALMALLIVATVLGNALVMLAFV
ADSSLRTQNNFFLLNLAISDFLVGAFCIPLYVPYVLTGRWTFGRGLCKLWLVVDYLLCTS
SAFNIVLISYDRFLSVTRAVSYRAQQGDTRRAVRKMLLVWVLAFLLYGPAILSWEYLSGG
SSIPEGHCYAEFFYNWYFLITASTLEFFTPFLSVTFFNLSIYLNIQRRTRLRLDGAREAA
GPEPPPEAQPSPPPPPGCWGCWQKGHGEAMPLHRYGVGEAAVGAEAGEATLGGGGGGGSV
ASPTSSSGSSSRGTERPRSLKRGSKPSASSASLEKRMKMVSQSFTQRFRLSRDRKVAKSL
AVIVSIFGLCWAPYTLLMIIRAACHGHCVPDYWYETSFWLLWANSAVNPVLYPLCHHSFR
RAFTKLLCPQKLKIQPHSSLEHCWK
|
|
|
|---|
| BDBM50139346 |
|---|
| n/a |
|---|
| Name | BDBM50139346 |
|---|
| Synonyms: | CHEMBL158714 | Furan-2-carboxylic acid {(R)-2-[4-(3-{3-fluoro-4-[5-(3-methyl-butyl)-[1,2,4]oxadiazol-3-yl]-phenoxy}-propyl)-[1,4]diazepan-1-yl]-1-methyl-2-oxo-ethyl}-amide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H38FN5O5 |
|---|
| Mol. Mass. | 555.6409 |
|---|
| SMILES | CC(C)CCc1nc(no1)-c1ccc(OCCCN2CCCN(CC2)C(=O)[C@@H](C)NC(=O)c2ccco2)cc1F |
|---|
| Structure | 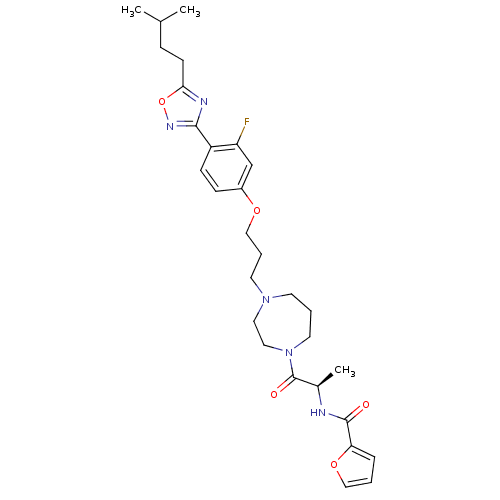
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gfesser, GA; Zhang, H; Dinges, J; Fox, GB; Pan, JB; Esbenshade, TA; Yao, BB; Witte, D; Miller, TR; Kang, CH; Krueger, KM; Bennani, YL; Hancock, AA; Faghih, R Structure-activity relationships of non-imidazole H(3) receptor ligands. Part 3: 5-Substituted 3-phenyl-1,2,4-oxadiazoles as potent antagonists. Bioorg Med Chem Lett14:673-6 (2004) [PubMed]
Gfesser, GA; Zhang, H; Dinges, J; Fox, GB; Pan, JB; Esbenshade, TA; Yao, BB; Witte, D; Miller, TR; Kang, CH; Krueger, KM; Bennani, YL; Hancock, AA; Faghih, R Structure-activity relationships of non-imidazole H(3) receptor ligands. Part 3: 5-Substituted 3-phenyl-1,2,4-oxadiazoles as potent antagonists. Bioorg Med Chem Lett14:673-6 (2004) [PubMed]