 Found 5294 hits with Last Name = 'witte' and Initial = 'd'
Found 5294 hits with Last Name = 'witte' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine receptor H3
(Dog) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50074629
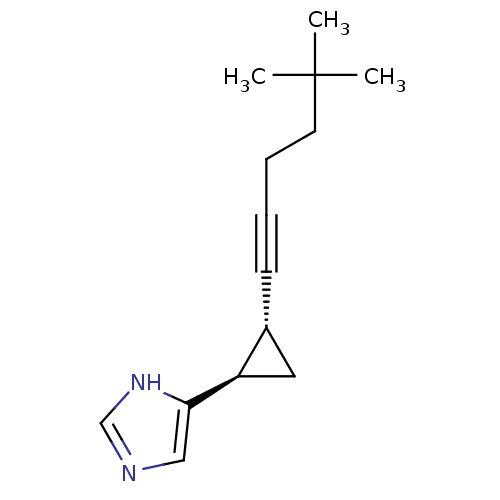
(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27210

((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](NC)C(C)C)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-18(2)26(30-5)27(33)32(7)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(6)19(3)23(29)10-11-25(22)29/h8,18-19,21-26,30H,9-17H2,1-7H3/t19-,21-,22+,23+,24-,25-,26+,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha methyl histamine from human cloned histamine H3 receptor expressed in C6 cells |
J Med Chem 52: 4640-9 (2009)
Article DOI: 10.1021/jm900480x
BindingDB Entry DOI: 10.7270/Q2Z31ZNT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27210

((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](NC)C(C)C)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-18(2)26(30-5)27(33)32(7)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(6)19(3)23(29)10-11-25(22)29/h8,18-19,21-26,30H,9-17H2,1-7H3/t19-,21-,22+,23+,24-,25-,26+,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27208
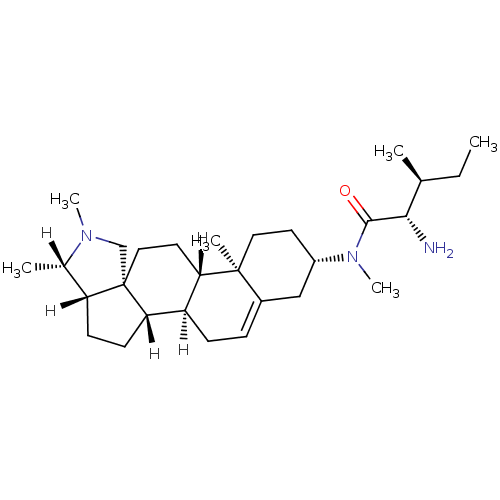
((2S,3S)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@@H](N)[C@@H](C)CC)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-7-18(2)26(30)27(33)32(6)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(5)19(3)23(29)10-11-25(22)29/h8,18-19,21-26H,7,9-17,30H2,1-6H3/t18-,19-,21-,22+,23+,24-,25-,26-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50074629

(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27209

((2R,3R)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](N)[C@H](C)CC)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-7-18(2)26(30)27(33)32(6)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(5)19(3)23(29)10-11-25(22)29/h8,18-19,21-26H,7,9-17,30H2,1-6H3/t18-,19+,21+,22-,23-,24+,25+,26-,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | -53.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(GUINEA PIG) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 39: 346-51 (1991)
BindingDB Entry DOI: 10.7270/Q2222S8S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158595
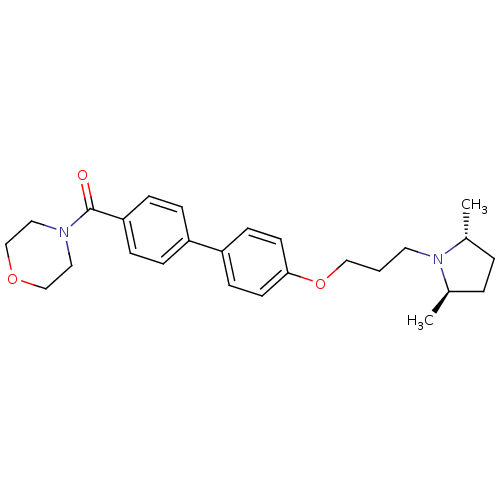
((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158595

((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha methyl histamine from human cloned histamine H3 receptor expressed in C6 cells |
J Med Chem 52: 4640-9 (2009)
Article DOI: 10.1021/jm900480x
BindingDB Entry DOI: 10.7270/Q2Z31ZNT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27208

((2S,3S)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@@H](N)[C@@H](C)CC)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-7-18(2)26(30)27(33)32(6)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(5)19(3)23(29)10-11-25(22)29/h8,18-19,21-26H,7,9-17,30H2,1-6H3/t18-,19-,21-,22+,23+,24-,25-,26-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50294063
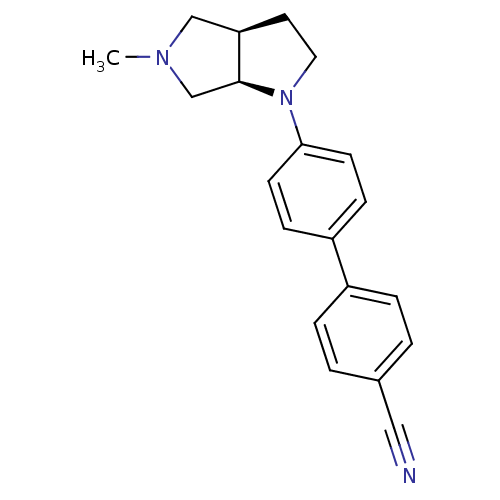
((3aR,6aR)-4'-(5-Methyl-hexahydro-pyrrolo[3,4-b]pyr...)Show SMILES CN1C[C@H]2CCN([C@H]2C1)c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H21N3/c1-22-13-18-10-11-23(20(18)14-22)19-8-6-17(7-9-19)16-4-2-15(12-21)3-5-16/h2-9,18,20H,10-11,13-14H2,1H3/t18-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha methyl histamine from human cloned histamine H3 receptor expressed in C6 cells |
J Med Chem 52: 4640-9 (2009)
Article DOI: 10.1021/jm900480x
BindingDB Entry DOI: 10.7270/Q2Z31ZNT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50158595

((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139346
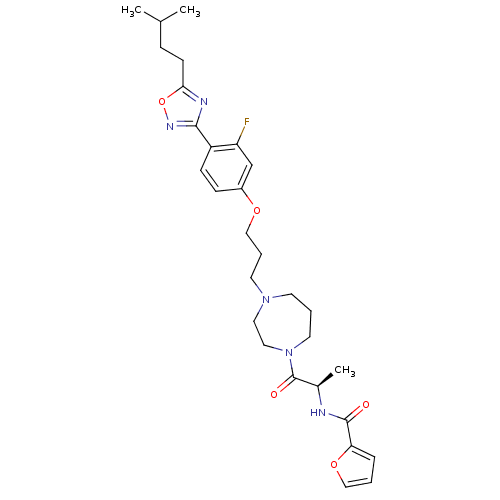
(CHEMBL158714 | Furan-2-carboxylic acid {(R)-2-[4-(...)Show SMILES CC(C)CCc1nc(no1)-c1ccc(OCCCN2CCCN(CC2)C(=O)[C@@H](C)NC(=O)c2ccco2)cc1F Show InChI InChI=1S/C29H38FN5O5/c1-20(2)8-11-26-32-27(33-40-26)23-10-9-22(19-24(23)30)38-18-6-13-34-12-5-14-35(16-15-34)29(37)21(3)31-28(36)25-7-4-17-39-25/h4,7,9-10,17,19-21H,5-6,8,11-16,18H2,1-3H3,(H,31,36)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139288
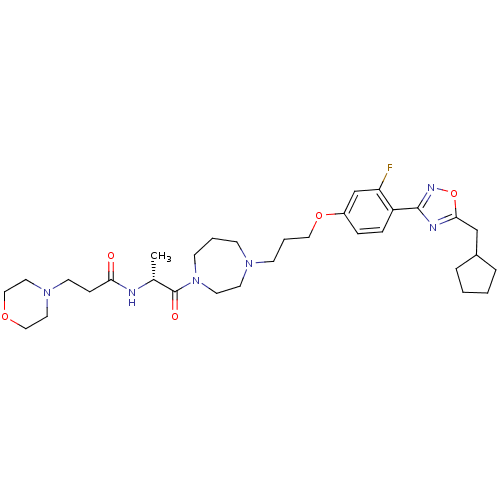
(CHEMBL158761 | N-[(R)-2-(4-{3-[4-(5-Cyclopentylmet...)Show SMILES C[C@@H](NC(=O)CCN1CCOCC1)C(=O)N1CCCN(CCCOc2ccc(-c3noc(CC4CCCC4)n3)c(F)c2)CC1 Show InChI InChI=1S/C32H47FN6O5/c1-24(34-29(40)10-14-38-17-20-42-21-18-38)32(41)39-13-4-11-37(15-16-39)12-5-19-43-26-8-9-27(28(33)23-26)31-35-30(44-36-31)22-25-6-2-3-7-25/h8-9,23-25H,2-7,10-22H2,1H3,(H,34,40)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50074629

(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50158601
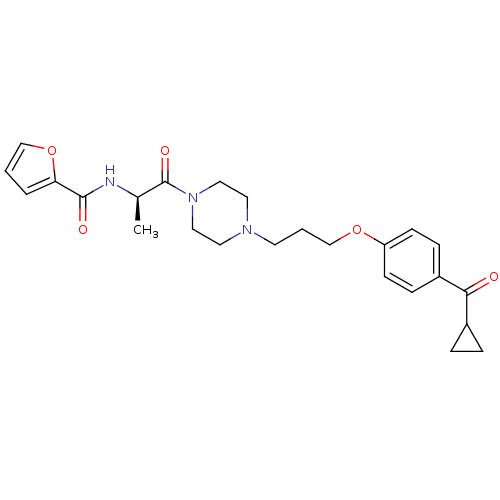
(A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)CC1 Show InChI InChI=1S/C25H31N3O5/c1-18(26-24(30)22-4-2-16-33-22)25(31)28-14-12-27(13-15-28)11-3-17-32-21-9-7-20(8-10-21)23(29)19-5-6-19/h2,4,7-10,16,18-19H,3,5-6,11-15,17H2,1H3,(H,26,30)/t18-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50158601

(A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)CC1 Show InChI InChI=1S/C25H31N3O5/c1-18(26-24(30)22-4-2-16-33-22)25(31)28-14-12-27(13-15-28)11-3-17-32-21-9-7-20(8-10-21)23(29)19-5-6-19/h2,4,7-10,16,18-19H,3,5-6,11-15,17H2,1H3,(H,26,30)/t18-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139326
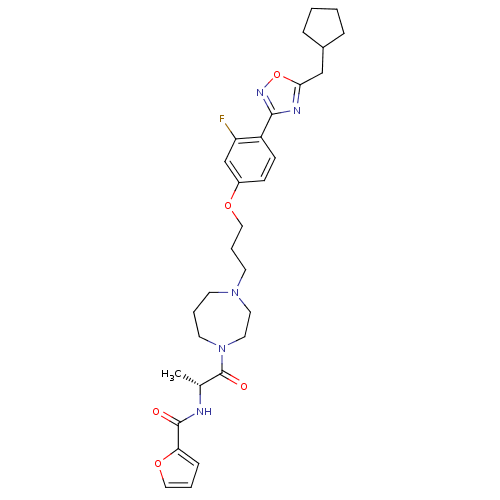
(CHEMBL349692 | Furan-2-carboxylic acid [(R)-2-(4-{...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCCN(CCCOc2ccc(-c3noc(CC4CCCC4)n3)c(F)c2)CC1 Show InChI InChI=1S/C30H38FN5O5/c1-21(32-29(37)26-9-4-17-40-26)30(38)36-14-5-12-35(15-16-36)13-6-18-39-23-10-11-24(25(31)20-23)28-33-27(41-34-28)19-22-7-2-3-8-22/h4,9-11,17,20-22H,2-3,5-8,12-16,18-19H2,1H3,(H,32,37)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139279

(CHEMBL161202 | Furan-2-carboxylic acid [(R)-2-(4-{...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCCN(CCCOc2ccc(-c3noc(n3)C3CCCC3)c(F)c2)CC1 Show InChI InChI=1S/C29H36FN5O5/c1-20(31-27(36)25-9-4-17-39-25)29(37)35-14-5-12-34(15-16-35)13-6-18-38-22-10-11-23(24(30)19-22)26-32-28(40-33-26)21-7-2-3-8-21/h4,9-11,17,19-21H,2-3,5-8,12-16,18H2,1H3,(H,31,36)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139289

(CHEMBL443817 | Furan-2-carboxylic acid [(R)-2-(4-{...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCCN(CCCOc2ccc(-c3noc(CCc4ccccc4)n3)c(F)c2)CC1 Show InChI InChI=1S/C32H36FN5O5/c1-23(34-31(39)28-10-5-20-42-28)32(40)38-17-6-15-37(18-19-38)16-7-21-41-25-12-13-26(27(33)22-25)30-35-29(43-36-30)14-11-24-8-3-2-4-9-24/h2-5,8-10,12-13,20,22-23H,6-7,11,14-19,21H2,1H3,(H,34,39)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119707
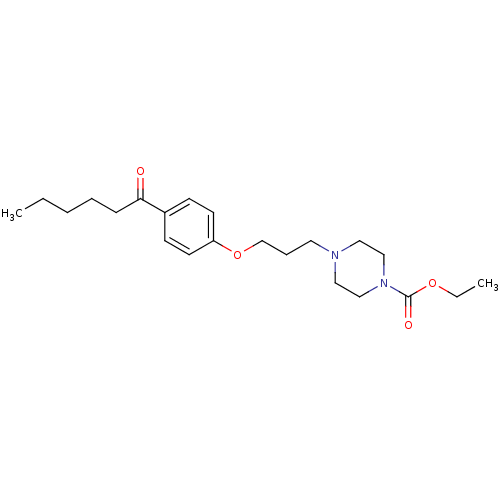
(4-[3-(4-Hexanoyl-phenoxy)-propyl]-piperazine-1-car...)Show InChI InChI=1S/C22H34N2O4/c1-3-5-6-8-21(25)19-9-11-20(12-10-19)28-18-7-13-23-14-16-24(17-15-23)22(26)27-4-2/h9-12H,3-8,13-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139303
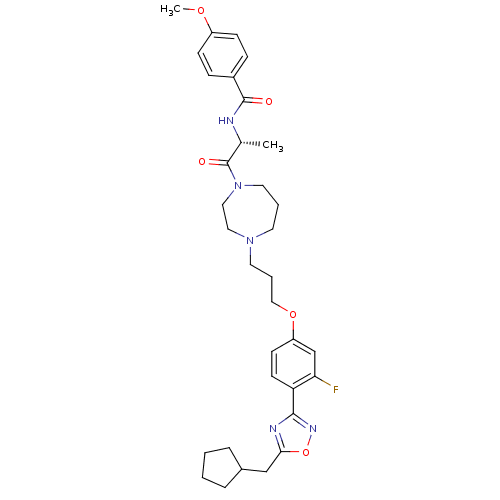
(CHEMBL350385 | N-[(R)-2-(4-{3-[4-(5-Cyclopentylmet...)Show SMILES COc1ccc(cc1)C(=O)N[C@H](C)C(=O)N1CCCN(CCCOc2ccc(-c3noc(CC4CCCC4)n3)c(F)c2)CC1 Show InChI InChI=1S/C33H42FN5O5/c1-23(35-32(40)25-9-11-26(42-2)12-10-25)33(41)39-17-5-15-38(18-19-39)16-6-20-43-27-13-14-28(29(34)22-27)31-36-30(44-37-31)21-24-7-3-4-8-24/h9-14,22-24H,3-8,15-21H2,1-2H3,(H,35,40)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139338
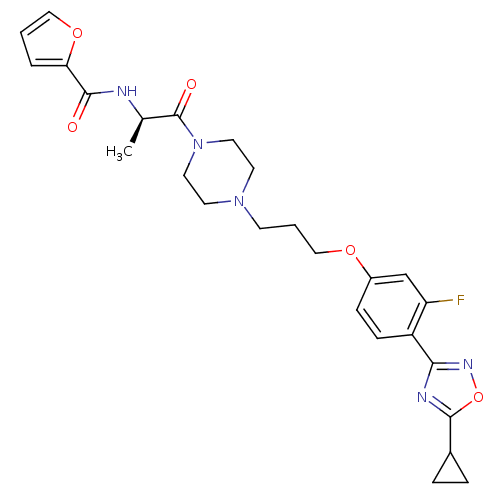
(CHEMBL157585 | Furan-2-carboxylic acid [(R)-2-(4-{...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCN(CCCOc2ccc(-c3noc(n3)C3CC3)c(F)c2)CC1 Show InChI InChI=1S/C26H30FN5O5/c1-17(28-24(33)22-4-2-14-36-22)26(34)32-12-10-31(11-13-32)9-3-15-35-19-7-8-20(21(27)16-19)23-29-25(37-30-23)18-5-6-18/h2,4,7-8,14,16-18H,3,5-6,9-13,15H2,1H3,(H,28,33)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139300
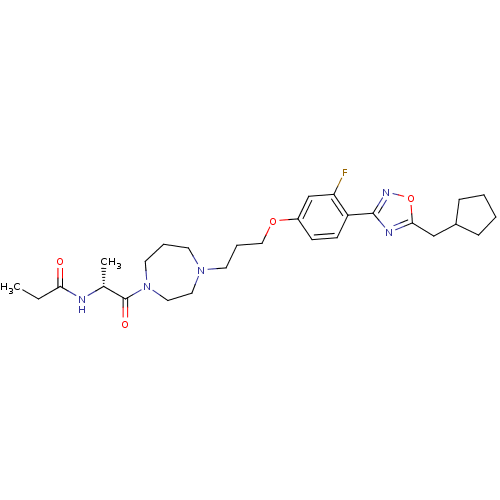
(CHEMBL162129 | N-[(R)-2-(4-{3-[4-(5-Cyclopentylmet...)Show SMILES CCC(=O)N[C@H](C)C(=O)N1CCCN(CCCOc2ccc(-c3noc(CC4CCCC4)n3)c(F)c2)CC1 Show InChI InChI=1S/C28H40FN5O4/c1-3-25(35)30-20(2)28(36)34-14-6-12-33(15-16-34)13-7-17-37-22-10-11-23(24(29)19-22)27-31-26(38-32-27)18-21-8-4-5-9-21/h10-11,19-21H,3-9,12-18H2,1-2H3,(H,30,35)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139350

(CHEMBL161743 | N-[(R)-2-(4-{3-[4-(5-Cyclopentylmet...)Show SMILES CC(C)CC(=O)N[C@H](C)C(=O)N1CCCN(CCCOc2ccc(-c3noc(CC4CCCC4)n3)c(F)c2)CC1 Show InChI InChI=1S/C30H44FN5O4/c1-21(2)18-27(37)32-22(3)30(38)36-14-6-12-35(15-16-36)13-7-17-39-24-10-11-25(26(31)20-24)29-33-28(40-34-29)19-23-8-4-5-9-23/h10-11,20-23H,4-9,12-19H2,1-3H3,(H,32,37)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139311
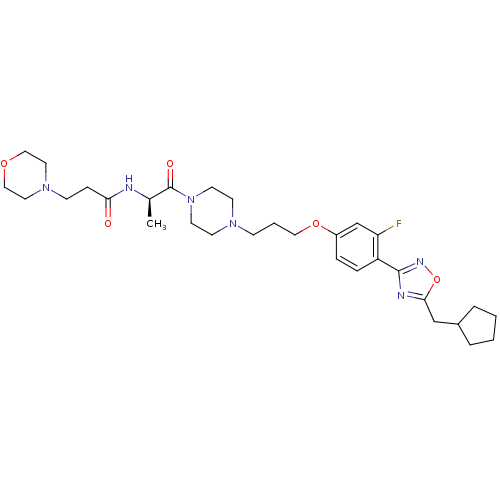
(CHEMBL162595 | N-[(R)-2-(4-{3-[4-(5-Cyclopentylmet...)Show SMILES C[C@@H](NC(=O)CCN1CCOCC1)C(=O)N1CCN(CCCOc2ccc(-c3noc(CC4CCCC4)n3)c(F)c2)CC1 Show InChI InChI=1S/C31H45FN6O5/c1-23(33-28(39)9-11-37-16-19-41-20-17-37)31(40)38-14-12-36(13-15-38)10-4-18-42-25-7-8-26(27(32)22-25)30-34-29(43-35-30)21-24-5-2-3-6-24/h7-8,22-24H,2-6,9-21H2,1H3,(H,33,39)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27212

((2S)-2-acetamido-N-methyl-3-phenyl-N-[(1R,2S,5S,6S...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](Cc4ccccc4)NC(C)=O)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C34H49N3O2/c1-22-28-13-14-30-27-12-11-25-20-26(15-17-33(25,3)29(27)16-18-34(28,30)21-36(22)4)37(5)32(39)31(35-23(2)38)19-24-9-7-6-8-10-24/h6-11,22,26-31H,12-21H2,1-5H3,(H,35,38)/t22-,26-,27+,28+,29-,30-,31-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.15 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139273
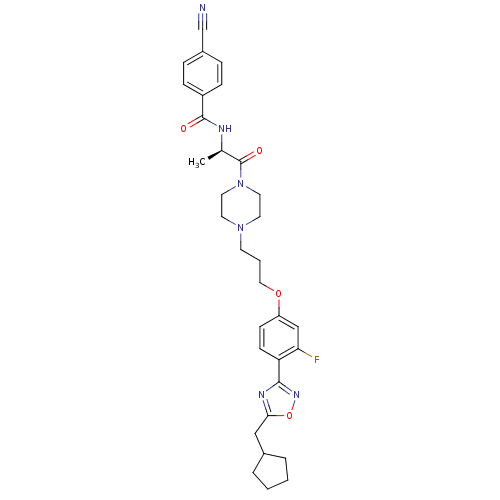
(2-(4-Cyano-phenyl)-N-[(R)-2-(4-{3-[4-(5-cyclopenty...)Show SMILES C[C@@H](NC(=O)c1ccc(cc1)C#N)C(=O)N1CCN(CCCOc2ccc(-c3noc(CC4CCCC4)n3)c(F)c2)CC1 Show InChI InChI=1S/C32H37FN6O4/c1-22(35-31(40)25-9-7-24(21-34)8-10-25)32(41)39-16-14-38(15-17-39)13-4-18-42-26-11-12-27(28(33)20-26)30-36-29(43-37-30)19-23-5-2-3-6-23/h7-12,20,22-23H,2-6,13-19H2,1H3,(H,35,40)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Dog) | BDBM50158595

((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139285

(CHEMBL351192 | N-[(R)-2-(4-{3-[4-(5-Cyclopentylmet...)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)C(=O)N1CCCN(CCCOc2ccc(-c3noc(CC4CCCC4)n3)c(F)c2)CC1 Show InChI InChI=1S/C32H39F2N5O4/c1-22(35-31(40)24-8-10-25(33)11-9-24)32(41)39-16-4-14-38(17-18-39)15-5-19-42-26-12-13-27(28(34)21-26)30-36-29(43-37-30)20-23-6-2-3-7-23/h8-13,21-23H,2-7,14-20H2,1H3,(H,35,40)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50139391

((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM86061
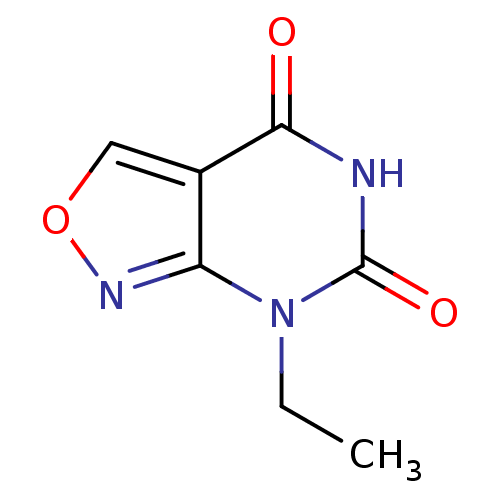
(A-304121)Show InChI InChI=1S/C7H7N3O3/c1-2-10-5-4(3-13-9-5)6(11)8-7(10)12/h3H,2H2,1H3,(H,8,11,12) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139333

(CHEMBL347909 | N-[(R)-2-(4-{3-[4-(5-Cyclopentylmet...)Show SMILES C[C@@H](NC(=O)c1ccc(O)cc1)C(=O)N1CCN(CCCOc2ccc(-c3noc(CC4CCCC4)n3)c(F)c2)CC1 Show InChI InChI=1S/C31H38FN5O5/c1-21(33-30(39)23-7-9-24(38)10-8-23)31(40)37-16-14-36(15-17-37)13-4-18-41-25-11-12-26(27(32)20-25)29-34-28(42-35-29)19-22-5-2-3-6-22/h7-12,20-22,38H,2-6,13-19H2,1H3,(H,33,39)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139348
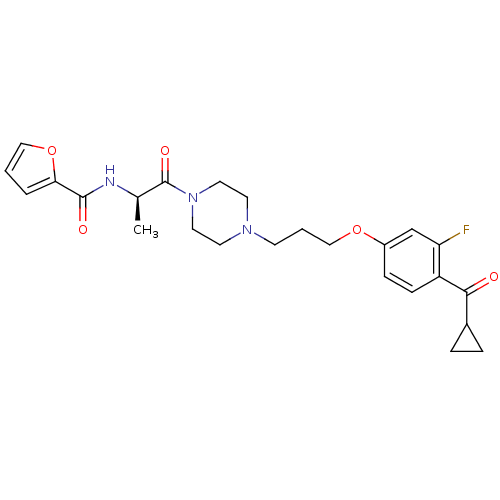
(CHEMBL161479 | Furan-2-carboxylic acid ((R)-2-{4-[...)Show SMILES C[C@@H](NC(=O)c1ccco1)C(=O)N1CCN(CCCOc2ccc(C(=O)C3CC3)c(F)c2)CC1 Show InChI InChI=1S/C25H30FN3O5/c1-17(27-24(31)22-4-2-14-34-22)25(32)29-12-10-28(11-13-29)9-3-15-33-19-7-8-20(21(26)16-19)23(30)18-5-6-18/h2,4,7-8,14,16-18H,3,5-6,9-13,15H2,1H3,(H,27,31)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50139265

(CHEMBL351101 | N-[(R)-2-(4-{3-[4-(5-Cyclopentylmet...)Show SMILES CC(C)C(=O)N[C@H](C)C(=O)N1CCCN(CCCOc2ccc(-c3noc(CC4CCCC4)n3)c(F)c2)CC1 Show InChI InChI=1S/C29H42FN5O4/c1-20(2)28(36)31-21(3)29(37)35-14-6-12-34(15-16-35)13-7-17-38-23-10-11-24(25(30)19-23)27-32-26(39-33-27)18-22-8-4-5-9-22/h10-11,19-22H,4-9,12-18H2,1-3H3,(H,31,36)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat histamine H3 receptor on rat cortical cells |
Bioorg Med Chem Lett 14: 673-6 (2004)
BindingDB Entry DOI: 10.7270/Q2H994MH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data