| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cyclin-dependent kinase 12 |
|---|
| Ligand | BDBM50573506 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2119654 (CHEMBL4828801) |
|---|
| IC50 | 69±n/a nM |
|---|
| Citation |  Jiang, B; Jiang, J; Kaltheuner, IH; Iniguez, AB; Anand, K; Ferguson, FM; Ficarro, SB; Seong, BKA; Greifenberg, AK; Dust, S; Kwiatkowski, NP; Marto, JA; Stegmaier, K; Zhang, T; Geyer, M; Gray, NS Structure-activity relationship study of THZ531 derivatives enables the discovery of BSJ-01-175 as a dual CDK12/13 covalent inhibitor with efficacy in Ewing sarcoma. Eur J Med Chem221:0 (2021) [PubMed] Article Jiang, B; Jiang, J; Kaltheuner, IH; Iniguez, AB; Anand, K; Ferguson, FM; Ficarro, SB; Seong, BKA; Greifenberg, AK; Dust, S; Kwiatkowski, NP; Marto, JA; Stegmaier, K; Zhang, T; Geyer, M; Gray, NS Structure-activity relationship study of THZ531 derivatives enables the discovery of BSJ-01-175 as a dual CDK12/13 covalent inhibitor with efficacy in Ewing sarcoma. Eur J Med Chem221:0 (2021) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cyclin-dependent kinase 12 |
|---|
| Name: | Cyclin-dependent kinase 12 |
|---|
| Synonyms: | 2.7.11.22 | 2.7.11.23 | CDC2-related protein kinase 7 | CDK12 | CDK12_HUMAN | CRK7 | CRKRS | Cdc2-related kinase, arginine/serine-rich | Cell division cycle 2-related protein kinase 7 | Cell division protein kinase 12 | Cyclin-dependent kinase 12 | KIAA0904 | hCDK12 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 164218.64 |
|---|
| Organism: | Homo sapiens |
|---|
| Description: | ChEMBL_117739 |
|---|
| Residue: | 1490 |
|---|
| Sequence: | MPNSERHGGKKDGSGGASGTLQPSSGGGSSNSRERHRLVSKHKRHKSKHSKDMGLVTPEA
ASLGTVIKPLVEYDDISSDSDTFSDDMAFKLDRRENDERRGSDRSDRLHKHRHHQHRRSR
DLLKAKQTEKEKSQEVSSKSGSMKDRISGSSKRSNEETDDYGKAQVAKSSSKESRSSKLH
KEKTRKERELKSGHKDRSKSHRKRETPKSYKTVDSPKRRSRSPHRKWSDSSKQDDSPSGA
SYGQDYDLSPSRSHTSSNYDSYKKSPGSTSRRQSVSPPYKEPSAYQSSTRSPSPYSRRQR
SVSPYSRRRSSSYERSGSYSGRSPSPYGRRRSSSPFLSKRSLSRSPLPSRKSMKSRSRSP
AYSRHSSSHSKKKRSSSRSRHSSISPVRLPLNSSLGAELSRKKKERAAAAAAAKMDGKES
KGSPVFLPRKENSSVEAKDSGLESKKLPRSVKLEKSAPDTELVNVTHLNTEVKNSSDTGK
VKLDENSEKHLVKDLKAQGTRDSKPIALKEEIVTPKETETSEKETPPPLPTIASPPPPLP
TTTPPPQTPPLPPLPPIPALPQQPPLPPSQPAFSQVPASSTSTLPPSTHSKTSAVSSQAN
SQPPVQVSVKTQVSVTAAIPHLKTSTLPPLPLPPLLPGDDDMDSPKETLPSKPVKKEKEQ
RTRHLLTDLPLPPELPGGDLSPPDSPEPKAITPPQQPYKKRPKICCPRYGERRQTESDWG
KRCVDKFDIIGIIGEGTYGQVYKAKDKDTGELVALKKVRLDNEKEGFPITAIREIKILRQ
LIHRSVVNMKEIVTDKQDALDFKKDKGAFYLVFEYMDHDLMGLLESGLVHFSEDHIKSFM
KQLMEGLEYCHKKNFLHRDIKCSNILLNNSGQIKLADFGLARLYNSEESRPYTNKVITLW
YRPPELLLGEERYTPAIDVWSCGCILGELFTKKPIFQANLELAQLELISRLCGSPCPAVW
PDVIKLPYFNTMKPKKQYRRRLREEFSFIPSAALDLLDHMLTLDPSKRCTAEQTLQSDFL
KDVELSKMAPPDLPHWQDCHELWSKKRRRQRQSGVVVEEPPPSKTSRKETTSGTSTEPVK
NSSPAPPQPAPGKVESGAGDAIGLADITQQLNQSELAVLLNLLQSQTDLSIPQMAQLLNI
HSNPEMQQQLEALNQSISALTEATSQQQDSETMAPEESLKEAPSAPVILPSAEQTTLEAS
STPADMQNILAVLLSQLMKTQEPAGSLEENNSDKNSGPQGPRRTPTMPQEEAAACPPHIL
PPEKRPPEPPGPPPPPPPPPLVEGDLSSAPQELNPAVTAALLQLLSQPEAEPPGHLPHEH
QALRPMEYSTRPRPNRTYGNTDGPETGFSAIDTDERNSGPALTESLVQTLVKNRTFSGSL
SHLGESSSYQGTGSVQFPGDQDLRFARVPLALHPVVGQPFLKAEGSSNSVVHAETKLQNY
GELGPGTTGASSSGAGLHWGGPTQSSAYGKLYRGPTRVPPRGGRGRGVPY
|
|
|
|---|
| BDBM50573506 |
|---|
| n/a |
|---|
| Name | BDBM50573506 |
|---|
| Synonyms: | CHEMBL4861833 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H30ClN7O2 |
|---|
| Mol. Mass. | 544.047 |
|---|
| SMILES | CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N1CC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| |
|---|
| Structure | 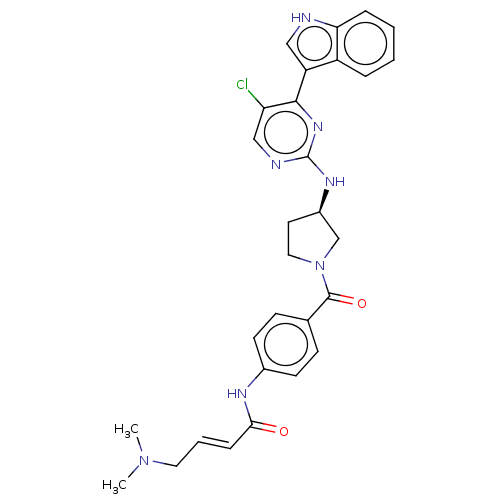
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jiang, B; Jiang, J; Kaltheuner, IH; Iniguez, AB; Anand, K; Ferguson, FM; Ficarro, SB; Seong, BKA; Greifenberg, AK; Dust, S; Kwiatkowski, NP; Marto, JA; Stegmaier, K; Zhang, T; Geyer, M; Gray, NS Structure-activity relationship study of THZ531 derivatives enables the discovery of BSJ-01-175 as a dual CDK12/13 covalent inhibitor with efficacy in Ewing sarcoma. Eur J Med Chem221:0 (2021) [PubMed] Article
Jiang, B; Jiang, J; Kaltheuner, IH; Iniguez, AB; Anand, K; Ferguson, FM; Ficarro, SB; Seong, BKA; Greifenberg, AK; Dust, S; Kwiatkowski, NP; Marto, JA; Stegmaier, K; Zhang, T; Geyer, M; Gray, NS Structure-activity relationship study of THZ531 derivatives enables the discovery of BSJ-01-175 as a dual CDK12/13 covalent inhibitor with efficacy in Ewing sarcoma. Eur J Med Chem221:0 (2021) [PubMed] Article