

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50526216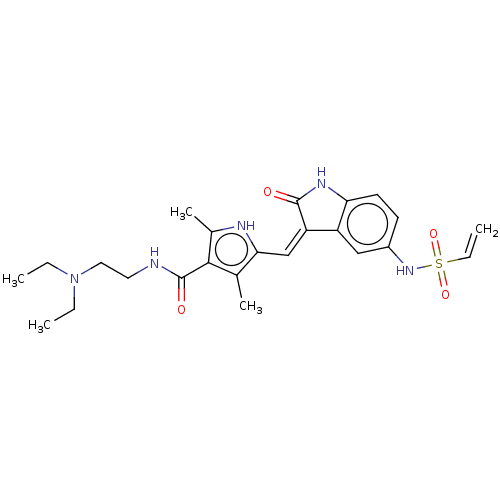 (CHEMBL4553725) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt Curated by ChEMBL | Assay Description Irreversible inhibition of recombinant human FLT3 D835Y mutant (571 to 993 residues) expressed in baculovirus expression system preincubated for 5 to... | J Med Chem 62: 2428-2446 (2019) Article DOI: 10.1021/acs.jmedchem.8b01714 BindingDB Entry DOI: 10.7270/Q26113R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50526217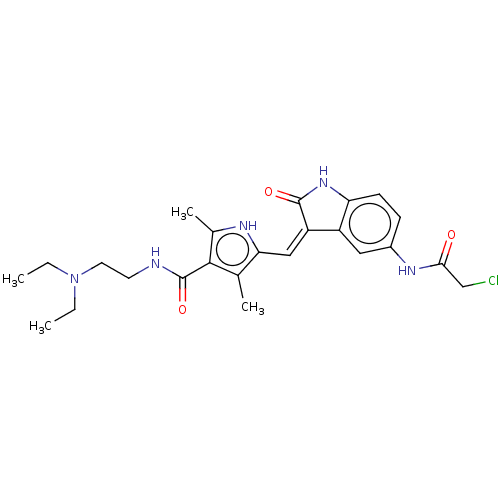 (CHEMBL4442997) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt Curated by ChEMBL | Assay Description Irreversible inhibition of recombinant human FLT3 D835Y mutant (571 to 993 residues) expressed in baculovirus expression system preincubated for 5 to... | J Med Chem 62: 2428-2446 (2019) Article DOI: 10.1021/acs.jmedchem.8b01714 BindingDB Entry DOI: 10.7270/Q26113R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188514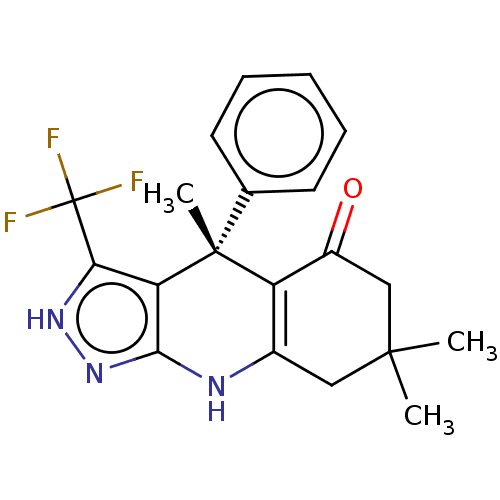 (4,7,7-trimethyl-4-phenyl-3-(trifluoromethyl)-2,4,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188512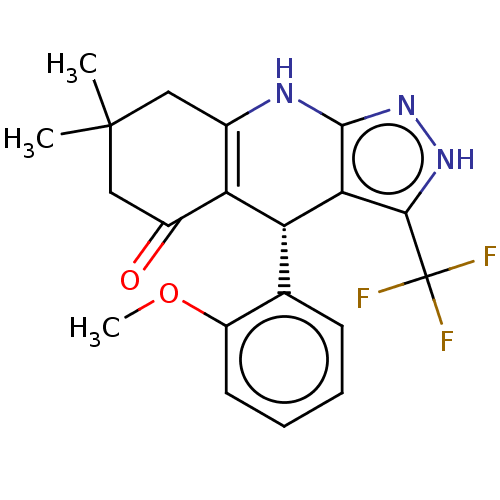 ((R)-4-(2-methoxyphenyl)-7,7-dimethyl-3-(trifluorom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188514 (4,7,7-trimethyl-4-phenyl-3-(trifluoromethyl)-2,4,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188515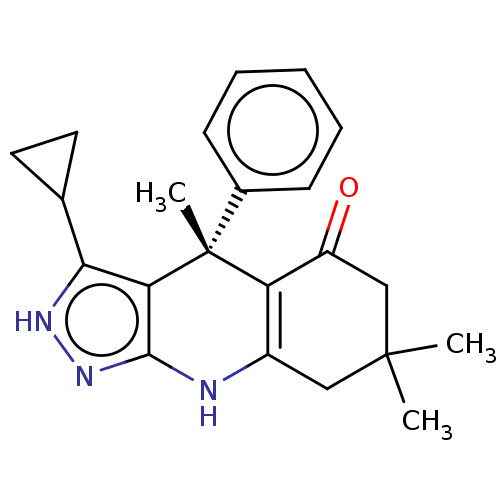 ((S)-3-cyclopropyl-4,7,7-trimethyl-4-phenyl-2,4,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188511 ((S)-3,7,7-Trimethyl-4-(2-(trifluoromethyl)phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM60933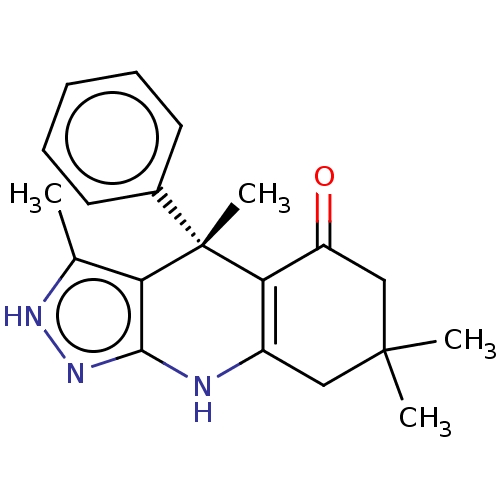 ((S)-3,4,7,7-tetramethyl-4-phenyl-2,4,6,7,8,9-hexah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188512 ((R)-4-(2-methoxyphenyl)-7,7-dimethyl-3-(trifluorom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188511 ((S)-3,7,7-Trimethyl-4-(2-(trifluoromethyl)phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM173197 (US10137122, Compound 53 | US9096594, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
The Broad Institute, Inc.; The General Hospital Corporation; Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | US Patent US9096594 (2015) BindingDB Entry DOI: 10.7270/Q21Z435K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM173197 (US10137122, Compound 53 | US9096594, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 25 |
The Broad Institute, Inc.; The General Hospital Corporation; Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | US Patent US9096594 (2015) BindingDB Entry DOI: 10.7270/Q21Z435K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50573506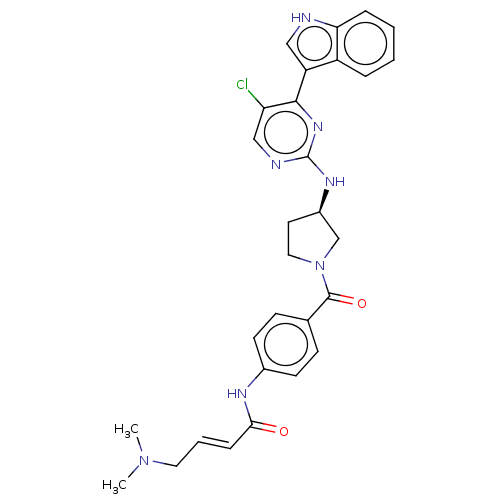 (CHEMBL4861833) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK9/Cyclin T1 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188505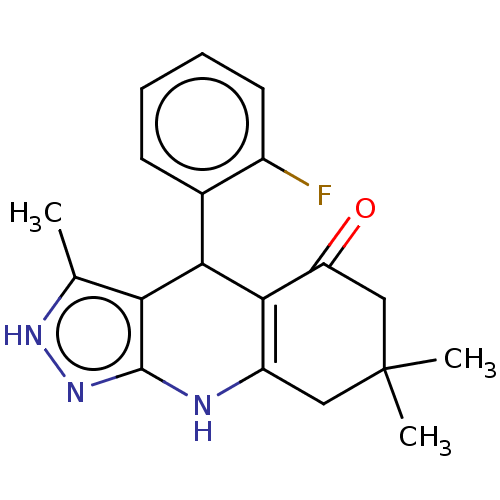 (4-(2-fluorophenyl)-3,7,7-trimethyl-2,4,6,7,8,9-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188515 ((S)-3-cyclopropyl-4,7,7-trimethyl-4-phenyl-2,4,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM60933 ((S)-3,4,7,7-tetramethyl-4-phenyl-2,4,6,7,8,9-hexah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM172196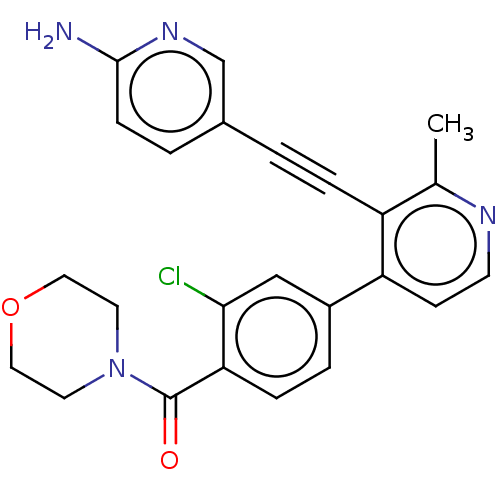 (US9090564, 10 | US9096594, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | 25 |
The Broad Institute, Inc.; The General Hospital Corporation; Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | US Patent US9096594 (2015) BindingDB Entry DOI: 10.7270/Q21Z435K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188509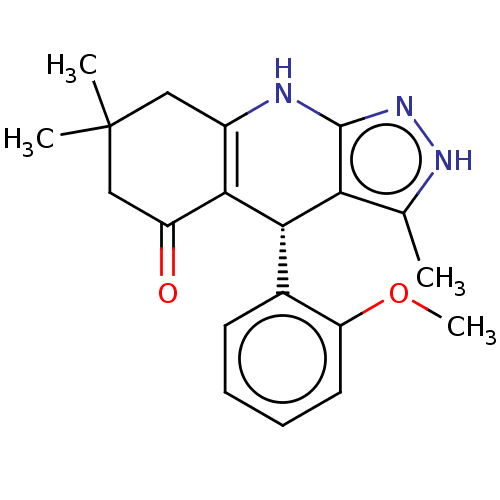 (BRD3937 | US10137122, Compound 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188508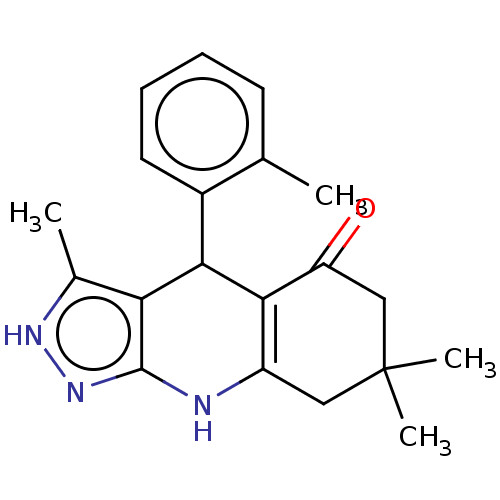 (3,7,7-Trimethyl-4-(o-tolyl)-2,4,6,7,8,9-hexahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50573512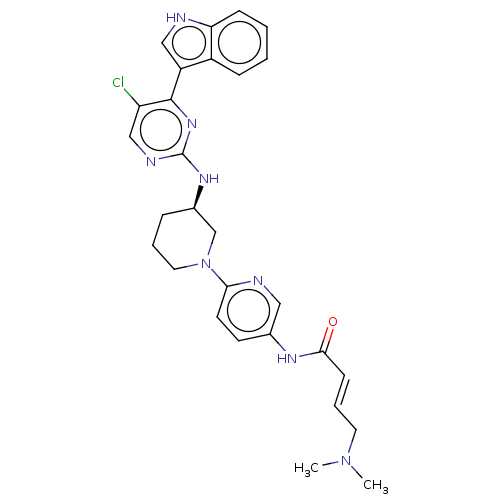 (CHEMBL4748005) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CDK12/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM173196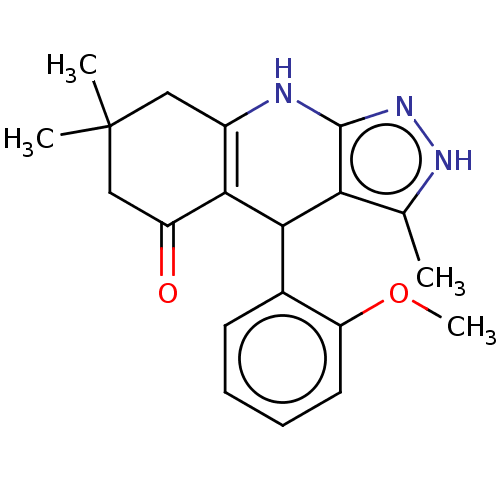 (4-(2-methoxyphenyl)-3,7,7-trimethyl-2,4,6,7,8,9-he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM50133388 (CHEMBL3632824) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK-3 alpha using Ulight-CFFKNIVTPRTPPPSQGK-amide substrate incubated for 60 mins by LANCE method relative to control | J Med Chem 58: 8907-19 (2015) Article DOI: 10.1021/acs.jmedchem.5b01200 BindingDB Entry DOI: 10.7270/Q2K64KW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50573506 (CHEMBL4861833) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin A (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 13/Cyclin-K (Homo sapiens (Human)) | BDBM50573506 (CHEMBL4861833) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CDK13/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM173196 (4-(2-methoxyphenyl)-3,7,7-trimethyl-2,4,6,7,8,9-he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188509 (BRD3937 | US10137122, Compound 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM173196 (4-(2-methoxyphenyl)-3,7,7-trimethyl-2,4,6,7,8,9-he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | 25 |
The Broad Institute, Inc.; The General Hospital Corporation; Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | US Patent US9096594 (2015) BindingDB Entry DOI: 10.7270/Q21Z435K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188506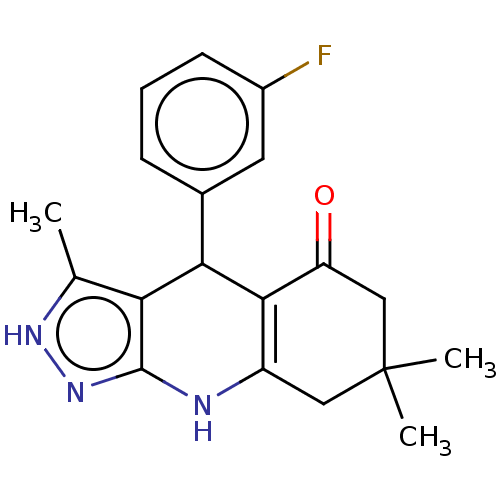 (4-(3-fluorophenyl)-3,7,7-trimethyl-2,4,6,7,8,9-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50573513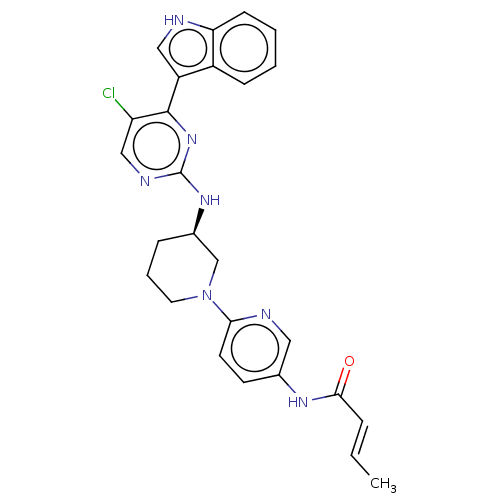 (CHEMBL4866757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CDK12/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50573506 (CHEMBL4861833) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CDK12/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM50573510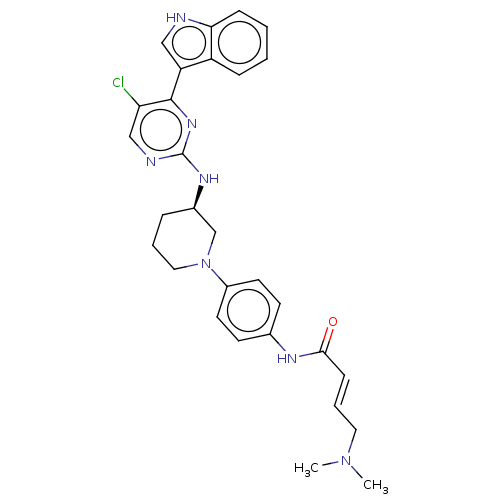 (CHEMBL4866812) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK7/Cyclin H (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM50573512 (CHEMBL4748005) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK7/Cyclin H (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188505 (4-(2-fluorophenyl)-3,7,7-trimethyl-2,4,6,7,8,9-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50573515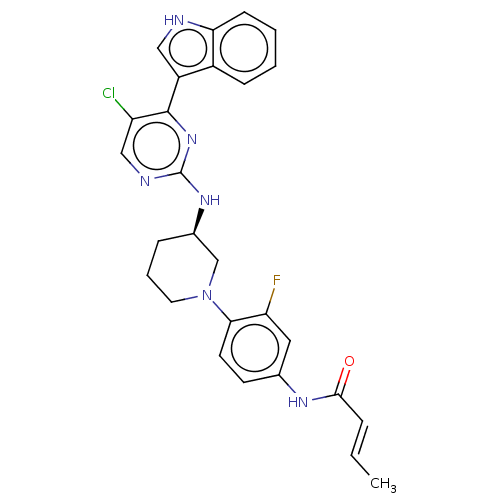 (CHEMBL4853887) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CDK12/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50573512 (CHEMBL4748005) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK9/Cyclin T1 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50573511 (CHEMBL4864697) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CDK12/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM303214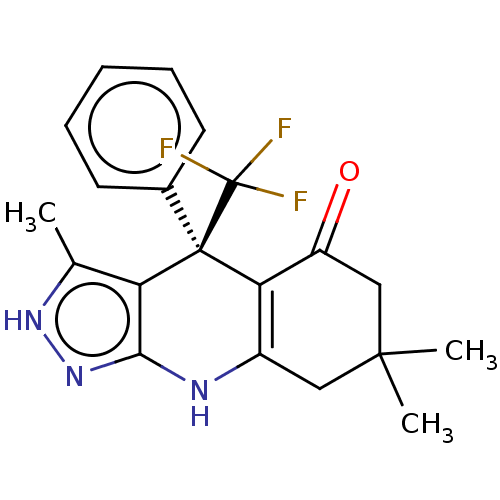 (US10137122, Compound 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188498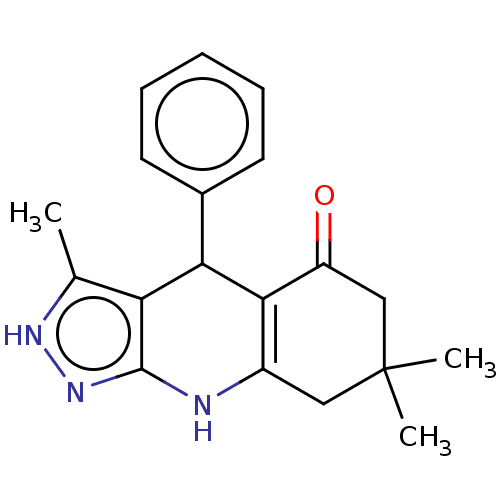 (3,7,7-trimethyl-4-phenyl-2,4,6,7,8,9-hexahydro-5H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188506 (4-(3-fluorophenyl)-3,7,7-trimethyl-2,4,6,7,8,9-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM303141 (US10137122, Compound 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM303141 (US10137122, Compound 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description 20 μL/well of 4.7 nM GSK3β and 7 μM peptide in KBA (250 mM HEPES (pH 7.5), 50 mM MgCl2, 5 mM EGTA, 0.05% BRIJ-35) were dispensed onto ... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM303226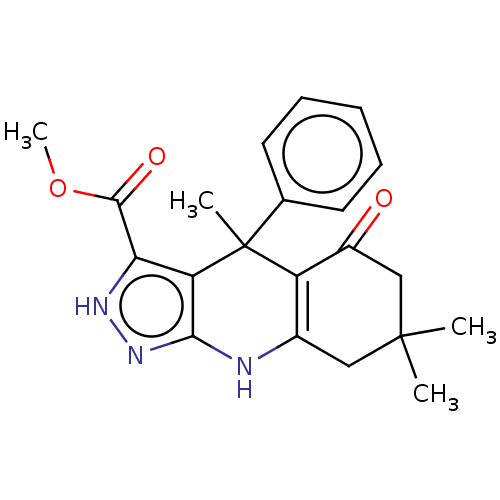 (US10137122, Compound 104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM303232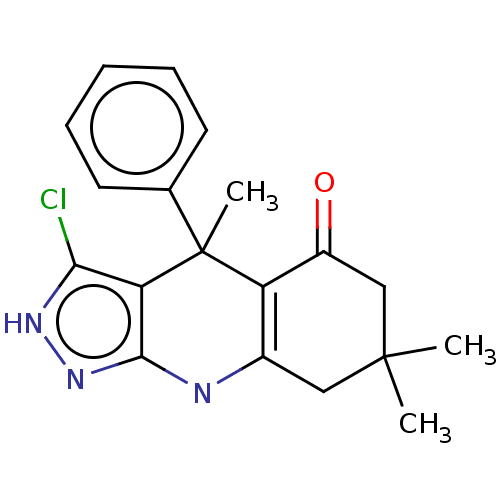 (US10137122, Compound 110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM303233 (US10137122, Compound 111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM303234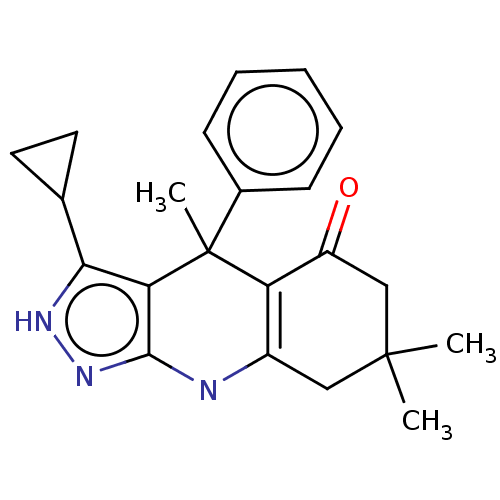 (US10137122, Compound 113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188515 ((S)-3-cyclopropyl-4,7,7-trimethyl-4-phenyl-2,4,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM303237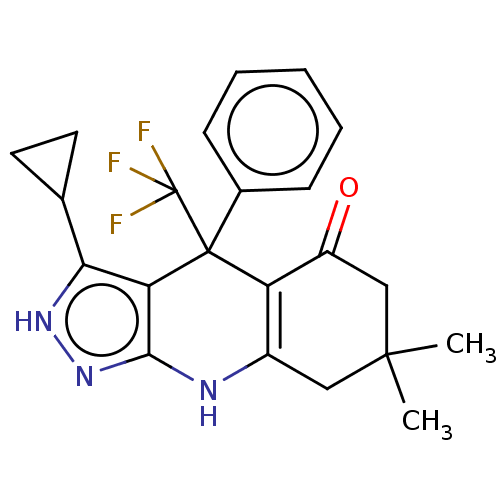 (US10137122, Compound 112) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM303238 (US10137122, Compound 116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM303240 (US10137122, Compound 117) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM303241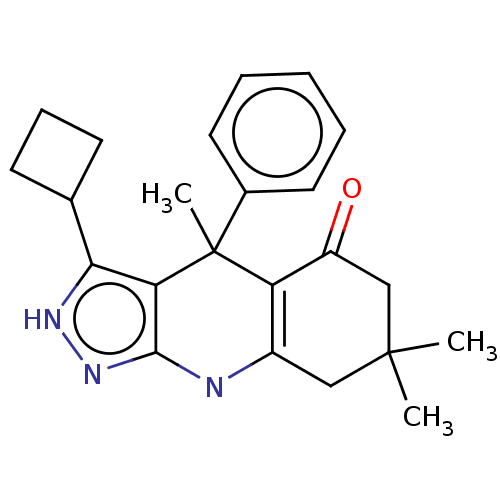 (US10137122, Compound 119) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute, Inc.; Dana-Farber Cancer Institute, Inc.; The General Hospital Corporation US Patent | Assay Description A solution of 4× inhibitor (5 μL), 4× substrate/ATP-Metal solution (5 μL), and 2× Kinase solution (10 μL) was prepared with assay buff... | US Patent US10137122 (2018) BindingDB Entry DOI: 10.7270/Q2DJ5HPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 437 total ) | Next | Last >> |