| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen synthase kinase-3 alpha |
|---|
| Ligand | BDBM188514 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Mobility Shift Microfluidics Assay |
|---|
| pH | 7.5±n/a |
|---|
| Temperature | 298.15±n/a K |
|---|
| IC50 | 0.4± 0.1 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Wagner, FF; Bishop, JA; Gale, JP; Shi, X; Walk, M; Ketterman, J; Patnaik, D; Barker, D; Walpita, D; Campbell, AJ; Nguyen, S; Lewis, M; Ross, L; We´wer, M; An, WF; Germain, AR; Nag, PP; Metkar, S; Kaya, T; Dandapani, S; Olson, DE; Barbe, AL; Lazzaro, F; Sacher, JR; Cheah, JH; Fei, D; Perez, J; Munoz, B; Palmer, M; Stegmaier, K; Schreiber, SL; Scolnick, E; Zhang, YL; Haggarty, SJ; Holson, EB; Pan, JQ Inhibitors of Glycogen Synthase Kinase 3 with Exquisite Kinome-Wide Selectivity and Their Functional Effects. ACS Chem Biol11:1952-63 (2016) [PubMed] Article Wagner, FF; Bishop, JA; Gale, JP; Shi, X; Walk, M; Ketterman, J; Patnaik, D; Barker, D; Walpita, D; Campbell, AJ; Nguyen, S; Lewis, M; Ross, L; We´wer, M; An, WF; Germain, AR; Nag, PP; Metkar, S; Kaya, T; Dandapani, S; Olson, DE; Barbe, AL; Lazzaro, F; Sacher, JR; Cheah, JH; Fei, D; Perez, J; Munoz, B; Palmer, M; Stegmaier, K; Schreiber, SL; Scolnick, E; Zhang, YL; Haggarty, SJ; Holson, EB; Pan, JQ Inhibitors of Glycogen Synthase Kinase 3 with Exquisite Kinome-Wide Selectivity and Their Functional Effects. ACS Chem Biol11:1952-63 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glycogen synthase kinase-3 alpha |
|---|
| Name: | Glycogen synthase kinase-3 alpha |
|---|
| Synonyms: | GSK-3 alpha | GSK3A | GSK3A_HUMAN | Glycogen synthase kinase 3 alpha (GSKalpha) | Glycogen synthase kinase-3 | Glycogen synthase kinase-3 alpha | Glycogen synthase kinase-3 alpha (GSK3 Alpha) | Glycogen synthase kinase-3 alpha (GSK3A) | Glycogen synthase kinase-3 alpha (GSK3alpha) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 50991.79 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P49840 |
|---|
| Residue: | 483 |
|---|
| Sequence: | MSGGGPSGGGPGGSGRARTSSFAEPGGGGGGGGGGPGGSASGPGGTGGGKASVGAMGGGV
GASSSGGGPGGSGGGGSGGPGAGTSFPPPGVKLGRDSGKVTTVVATLGQGPERSQEVAYT
DIKVIGNGSFGVVYQARLAETRELVAIKKVLQDKRFKNRELQIMRKLDHCNIVRLRYFFY
SSGEKKDELYLNLVLEYVPETVYRVARHFTKAKLTIPILYVKVYMYQLFRSLAYIHSQGV
CHRDIKPQNLLVDPDTAVLKLCDFGSAKQLVRGEPNVSYICSRYYRAPELIFGATDYTSS
IDVWSAGCVLAELLLGQPIFPGDSGVDQLVEIIKVLGTPTREQIREMNPNYTEFKFPQIK
AHPWTKVFKSRTPPEAIALCSSLLEYTPSSRLSPLEACAHSFFDELRCLGTQLPNNRPLP
PLFNFSAGELSIQPSLNAILIPPHLRSPAGTTTLTPSSQALTETPTSSDWQSTDATPTLT
NSS
|
|
|
|---|
| BDBM188514 |
|---|
| n/a |
|---|
| Name | BDBM188514 |
|---|
| Synonyms: | 4,7,7-trimethyl-4-phenyl-3-(trifluoromethyl)-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]quinolin-5-one (BRD1652) | US10137122, Compound 70 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H20F3N3O |
|---|
| Mol. Mass. | 375.3875 |
|---|
| SMILES | CC1(C)CC(=O)C2=C(C1)Nc1n[nH]c(c1[C@]2(C)c1ccccc1)C(F)(F)F |r,c:6| |
|---|
| Structure | 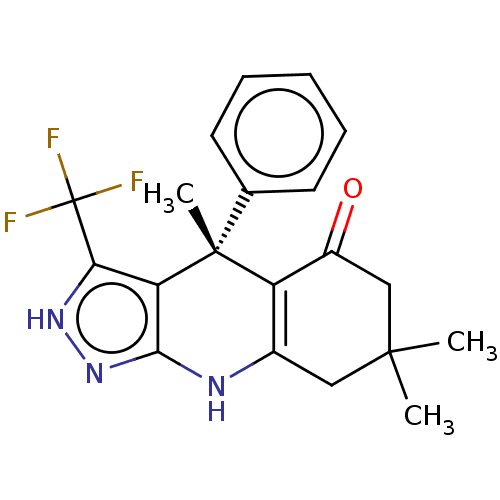
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wagner, FF; Bishop, JA; Gale, JP; Shi, X; Walk, M; Ketterman, J; Patnaik, D; Barker, D; Walpita, D; Campbell, AJ; Nguyen, S; Lewis, M; Ross, L; We´wer, M; An, WF; Germain, AR; Nag, PP; Metkar, S; Kaya, T; Dandapani, S; Olson, DE; Barbe, AL; Lazzaro, F; Sacher, JR; Cheah, JH; Fei, D; Perez, J; Munoz, B; Palmer, M; Stegmaier, K; Schreiber, SL; Scolnick, E; Zhang, YL; Haggarty, SJ; Holson, EB; Pan, JQ Inhibitors of Glycogen Synthase Kinase 3 with Exquisite Kinome-Wide Selectivity and Their Functional Effects. ACS Chem Biol11:1952-63 (2016) [PubMed] Article
Wagner, FF; Bishop, JA; Gale, JP; Shi, X; Walk, M; Ketterman, J; Patnaik, D; Barker, D; Walpita, D; Campbell, AJ; Nguyen, S; Lewis, M; Ross, L; We´wer, M; An, WF; Germain, AR; Nag, PP; Metkar, S; Kaya, T; Dandapani, S; Olson, DE; Barbe, AL; Lazzaro, F; Sacher, JR; Cheah, JH; Fei, D; Perez, J; Munoz, B; Palmer, M; Stegmaier, K; Schreiber, SL; Scolnick, E; Zhang, YL; Haggarty, SJ; Holson, EB; Pan, JQ Inhibitors of Glycogen Synthase Kinase 3 with Exquisite Kinome-Wide Selectivity and Their Functional Effects. ACS Chem Biol11:1952-63 (2016) [PubMed] Article