

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014480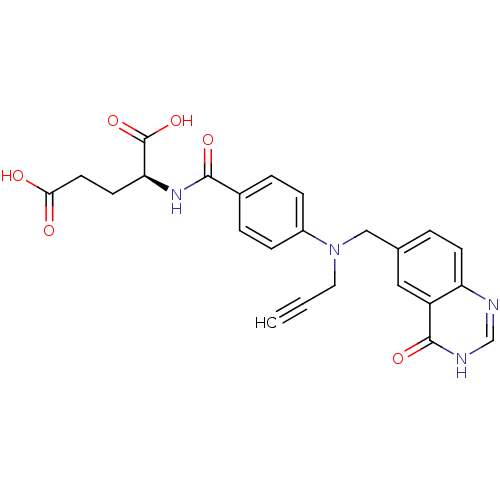 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of thymidylate synthase | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50014480 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Inhibitory activity against Dihydrofolate reductase in rat liver | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188514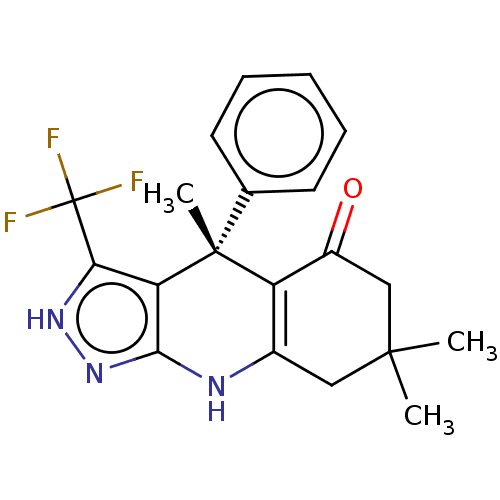 (4,7,7-trimethyl-4-phenyl-3-(trifluoromethyl)-2,4,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188512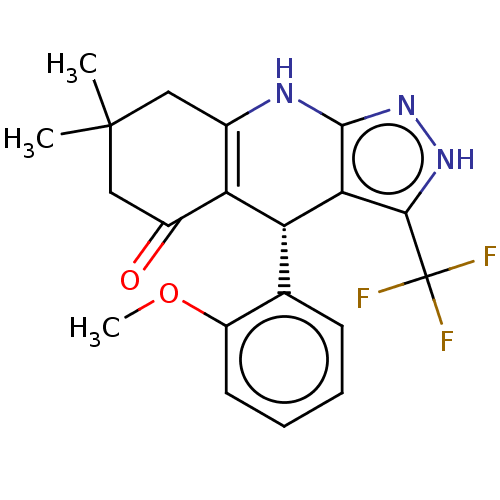 ((R)-4-(2-methoxyphenyl)-7,7-dimethyl-3-(trifluorom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188514 (4,7,7-trimethyl-4-phenyl-3-(trifluoromethyl)-2,4,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188515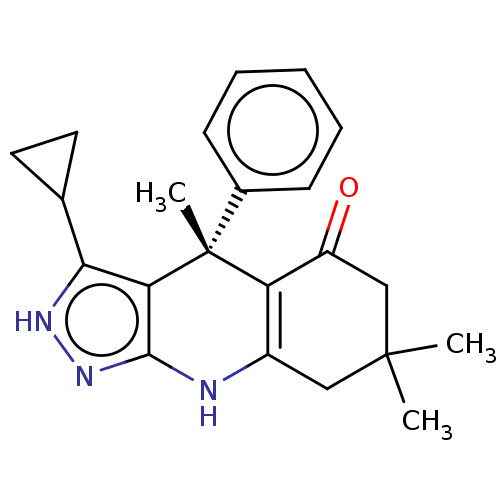 ((S)-3-cyclopropyl-4,7,7-trimethyl-4-phenyl-2,4,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188511 ((S)-3,7,7-Trimethyl-4-(2-(trifluoromethyl)phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM60933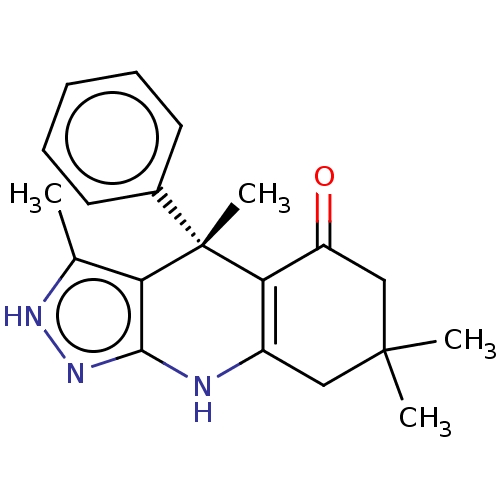 ((S)-3,4,7,7-tetramethyl-4-phenyl-2,4,6,7,8,9-hexah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014498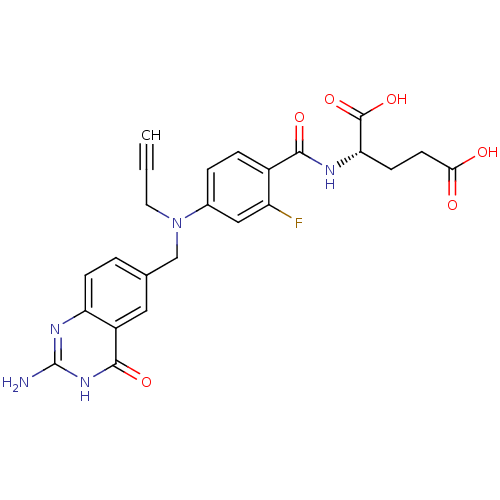 ((S)-2-(4-(((2-amino-4-oxo-3,4-dihydroquinazolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188512 ((R)-4-(2-methoxyphenyl)-7,7-dimethyl-3-(trifluorom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188511 ((S)-3,7,7-Trimethyl-4-(2-(trifluoromethyl)phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM173197 (US10137122, Compound 53 | US9096594, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
The Broad Institute, Inc.; The General Hospital Corporation; Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | US Patent US9096594 (2015) BindingDB Entry DOI: 10.7270/Q21Z435K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM173197 (US10137122, Compound 53 | US9096594, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 25 |
The Broad Institute, Inc.; The General Hospital Corporation; Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | US Patent US9096594 (2015) BindingDB Entry DOI: 10.7270/Q21Z435K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188505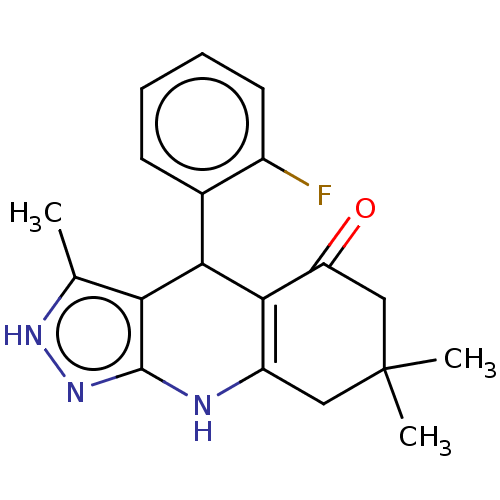 (4-(2-fluorophenyl)-3,7,7-trimethyl-2,4,6,7,8,9-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188515 ((S)-3-cyclopropyl-4,7,7-trimethyl-4-phenyl-2,4,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006689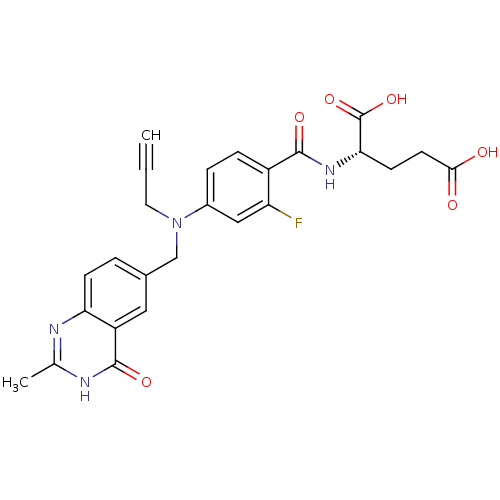 ((S)-2-(2-fluoro-4-(((2-methyl-4-oxo-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006689 ((S)-2-(2-fluoro-4-(((2-methyl-4-oxo-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase from L1210 cells | J Med Chem 35: 2321-7 (1992) BindingDB Entry DOI: 10.7270/Q2XW4HRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006689 ((S)-2-(2-fluoro-4-(((2-methyl-4-oxo-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against thymidylate synthase (TS) from L1210 mouse leukemia cells | J Med Chem 35: 2761-8 (1992) BindingDB Entry DOI: 10.7270/Q2K9385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of thymidylate synthase | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50004387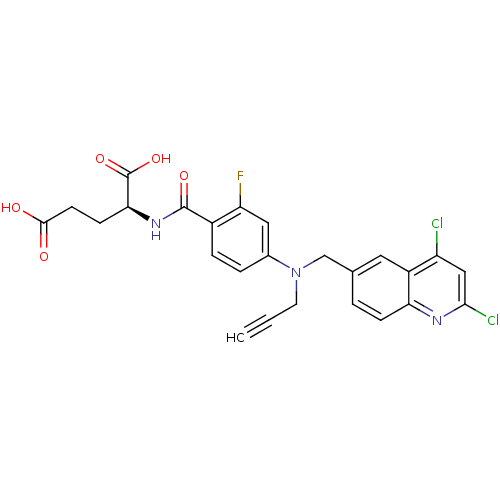 (2-{4-[(2,4-Dichloro-quinolin-6-ylmethyl)-prop-2-yn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 35: 2761-8 (1992) BindingDB Entry DOI: 10.7270/Q2K9385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM60933 ((S)-3,4,7,7-tetramethyl-4-phenyl-2,4,6,7,8,9-hexah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM172196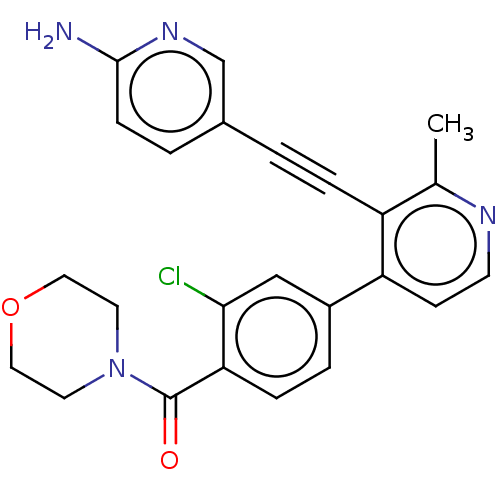 (US9090564, 10 | US9096594, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | 25 |
The Broad Institute, Inc.; The General Hospital Corporation; Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | US Patent US9096594 (2015) BindingDB Entry DOI: 10.7270/Q21Z435K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188509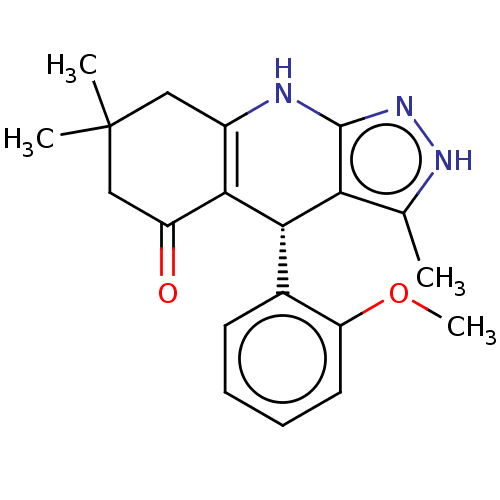 (BRD3937 | US10137122, Compound 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188508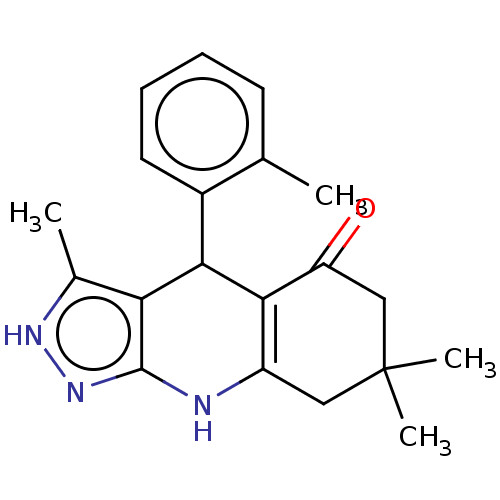 (3,7,7-Trimethyl-4-(o-tolyl)-2,4,6,7,8,9-hexahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014496 ((S)-2-(2-fluoro-4-(((2-methoxy-4-oxo-3,4-dihydroqu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM173196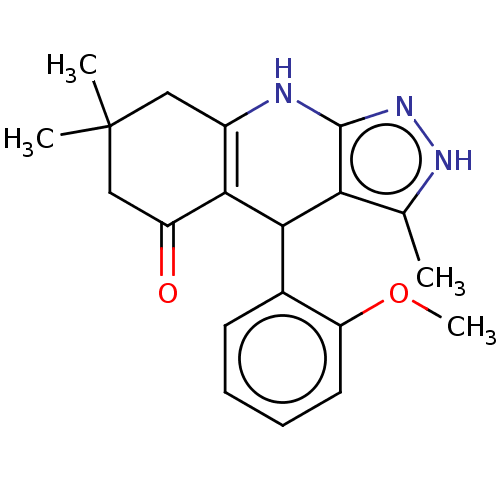 (4-(2-methoxyphenyl)-3,7,7-trimethyl-2,4,6,7,8,9-he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50012036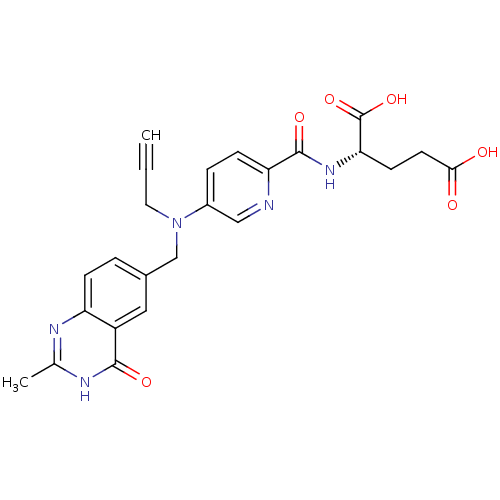 ((S)-2-(5-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of partially purified L1210 thymidylate synthase (TS). | J Med Chem 34: 1594-605 (1991) BindingDB Entry DOI: 10.7270/Q22N52VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of partially purified L1210 thymidylate synthase (TS). | J Med Chem 34: 1594-605 (1991) BindingDB Entry DOI: 10.7270/Q22N52VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Binding affinity against Thymidylate synthase was measured in vitro | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the compound against L1210 thymidylate synthase (TS) | J Med Chem 34: 2209-18 (1991) BindingDB Entry DOI: 10.7270/Q2D799D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase from L1210 cells | J Med Chem 35: 2321-7 (1992) BindingDB Entry DOI: 10.7270/Q2XW4HRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014501 ((S)-2-(2-fluoro-4-((2-fluoroethyl)((2-methyl-4-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014506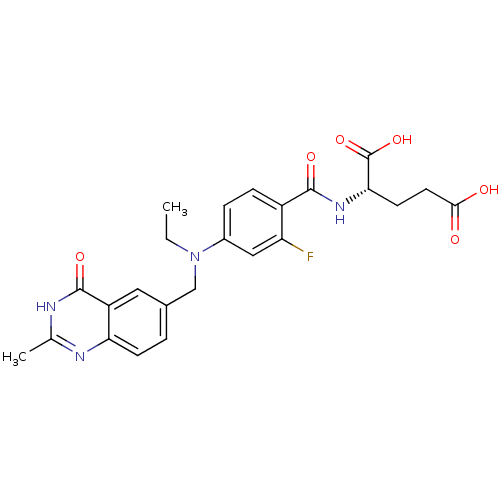 ((S)-2-(4-(ethyl((2-methyl-4-oxo-3,4-dihydroquinazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially purified thymidylate synthase (TS) | J Med Chem 33: 3072-8 (1990) BindingDB Entry DOI: 10.7270/Q2QC02G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50004381 (2-{4-[(2-Amino-4-chloro-quinolin-6-ylmethyl)-prop-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 35: 2761-8 (1992) BindingDB Entry DOI: 10.7270/Q2K9385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50006687 ((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM173196 (4-(2-methoxyphenyl)-3,7,7-trimethyl-2,4,6,7,8,9-he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM188509 (BRD3937 | US10137122, Compound 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014497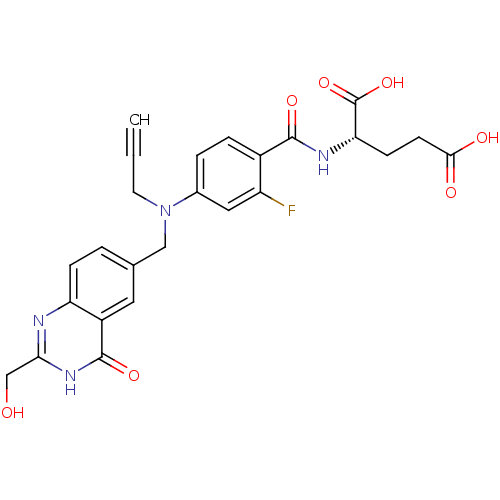 ((S)-2-(2-fluoro-4-(((2-(hydroxymethyl)-4-oxo-3,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014494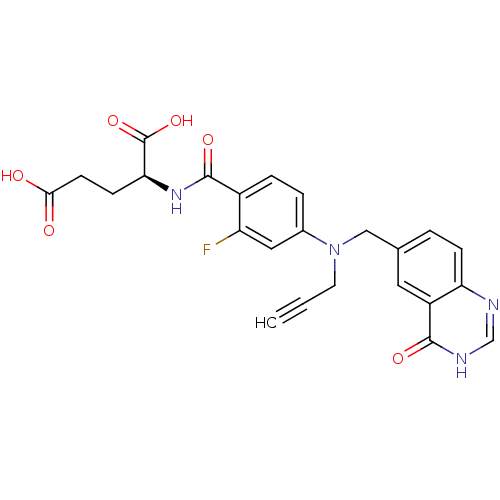 ((S)-2-(2-fluoro-4-(((4-oxo-3,4-dihydroquinazolin-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM173196 (4-(2-methoxyphenyl)-3,7,7-trimethyl-2,4,6,7,8,9-he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | 25 |
The Broad Institute, Inc.; The General Hospital Corporation; Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | US Patent US9096594 (2015) BindingDB Entry DOI: 10.7270/Q21Z435K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM188506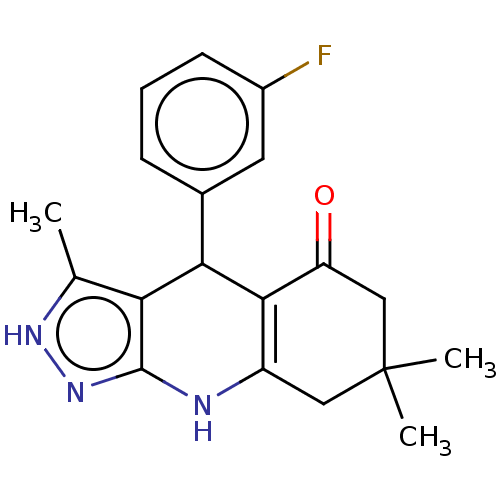 (4-(3-fluorophenyl)-3,7,7-trimethyl-2,4,6,7,8,9-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Harvard Medical School | Assay Description Purified GSK3beta was incubated with tested compounds in doses in the presence of 4.3 uM of ATP and 1.5uM peptide substrate (Peptide 15, Caliper, MA)... | ACS Chem Biol 11: 1952-63 (2016) Article DOI: 10.1021/acschembio.6b00306 BindingDB Entry DOI: 10.7270/Q20000W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50004395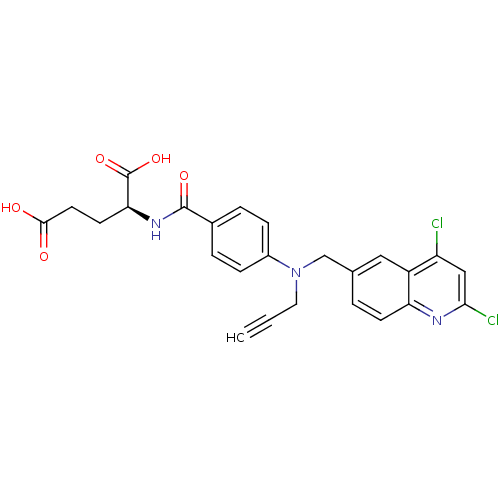 (2-{4-[(2,4-Dichloro-quinolin-6-ylmethyl)-prop-2-yn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 35: 2761-8 (1992) BindingDB Entry DOI: 10.7270/Q2K9385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50004372 (2-{4-[(4-Chloro-2-methyl-quinolin-6-ylmethyl)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against thymidylate synthase from L1210 mouse leukemia cells | J Med Chem 35: 2761-8 (1992) BindingDB Entry DOI: 10.7270/Q2K9385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 497 total ) | Next | Last >> |