| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Kynurenine 3-monooxygenase |
|---|
| Ligand | BDBM50576347 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2127057 (CHEMBL4836402) |
|---|
| IC50 | 6.1±n/a nM |
|---|
| Citation |  Tsuboi, K; Kimura, H; Nakatsuji, Y; Kassai, M; Deai, Y; Isobe, Y Discovery of N-(6-(5-fluoro-2-(piperidin-1-yl)phenyl)pyridazin-3-yl)-1-(tetrahydro-2H-pyran-4-yl)methanesulfonamide as a brain-permeable and metabolically stable kynurenine monooxygenase inhibitor. Bioorg Med Chem Lett44:0 (2021) [PubMed] Article Tsuboi, K; Kimura, H; Nakatsuji, Y; Kassai, M; Deai, Y; Isobe, Y Discovery of N-(6-(5-fluoro-2-(piperidin-1-yl)phenyl)pyridazin-3-yl)-1-(tetrahydro-2H-pyran-4-yl)methanesulfonamide as a brain-permeable and metabolically stable kynurenine monooxygenase inhibitor. Bioorg Med Chem Lett44:0 (2021) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Kynurenine 3-monooxygenase |
|---|
| Name: | Kynurenine 3-monooxygenase |
|---|
| Synonyms: | KMO | KMO_HUMAN |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 55831.03 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1487479 |
|---|
| Residue: | 486 |
|---|
| Sequence: | MDSSVIQRKKVAVIGGGLVGSLQACFLAKRNFQIDVYEAREDTRVATFTRGRSINLALSH
RGRQALKAVGLEDQIVSQGIPMRARMIHSLSGKKSAIPYGTKSQYILSVSRENLNKDLLT
AAEKYPNVKMHFNHRLLKCNPEEGMITVLGSDKVPKDVTCDLIVGCDGAYSTVRSHLMKK
PRFDYSQQYIPHGYMELTIPPKNGDYAMEPNYLHIWPRNTFMMIALPNMNKSFTCTLFMP
FEEFEKLLTSNDVVDFFQKYFPDAIPLIGEKLLVQDFFLLPAQPMISVKCSSFHFKSHCV
LLGDAAHAIVPFFGQGMNAGFEDCLVFDELMDKFSNDLSLCLPVFSRLRIPDDHAISDLS
MYNYIEMRAHVNSSWFIFQKNMERFLHAIMPSTFIPLYTMVTFSRIRYHEAVQRWHWQKK
VINKGLFFLGSLIAISSTYLLIHYMSPRSFLRLRRPWNWIAHFRNTTCFPAKAVDSLEQI
SNLISR
|
|
|
|---|
| BDBM50576347 |
|---|
| n/a |
|---|
| Name | BDBM50576347 |
|---|
| Synonyms: | CHEMBL4849479 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H23FN4O2S |
|---|
| Mol. Mass. | 426.507 |
|---|
| SMILES | Cc1ccc(cc1)S(=O)(=O)Nc1ccc(nn1)-c1c(F)cccc1N1CCCCC1 |
|---|
| Structure | 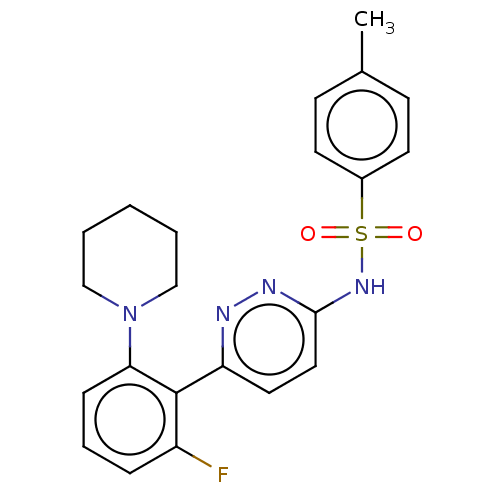
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tsuboi, K; Kimura, H; Nakatsuji, Y; Kassai, M; Deai, Y; Isobe, Y Discovery of N-(6-(5-fluoro-2-(piperidin-1-yl)phenyl)pyridazin-3-yl)-1-(tetrahydro-2H-pyran-4-yl)methanesulfonamide as a brain-permeable and metabolically stable kynurenine monooxygenase inhibitor. Bioorg Med Chem Lett44:0 (2021) [PubMed] Article
Tsuboi, K; Kimura, H; Nakatsuji, Y; Kassai, M; Deai, Y; Isobe, Y Discovery of N-(6-(5-fluoro-2-(piperidin-1-yl)phenyl)pyridazin-3-yl)-1-(tetrahydro-2H-pyran-4-yl)methanesulfonamide as a brain-permeable and metabolically stable kynurenine monooxygenase inhibitor. Bioorg Med Chem Lett44:0 (2021) [PubMed] Article