

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010335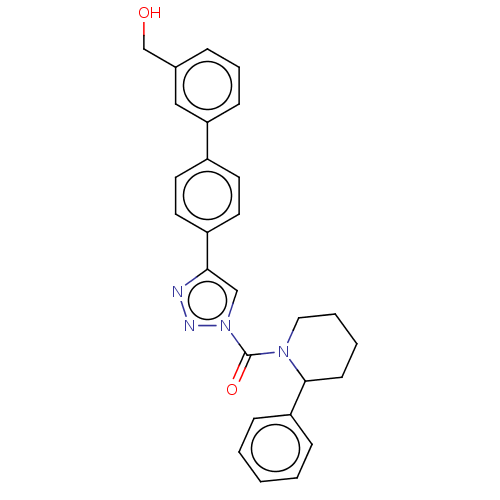 (CHEMBL3263577) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In situ inhibition of ABHD6 in mouse Neuro2a cells after 4 hrs by ABPP-SILAC-based LC-MS/MS analysis | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010336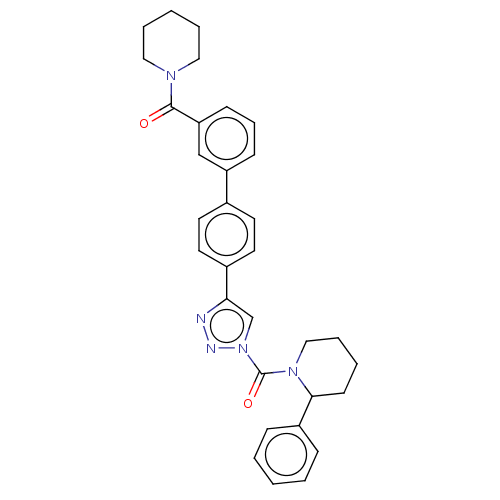 (CHEMBL3263579) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In situ inhibition of ABHD6 in mouse Neuro2a cells after 4 hrs by ABPP-SILAC-based LC-MS/MS analysis | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010337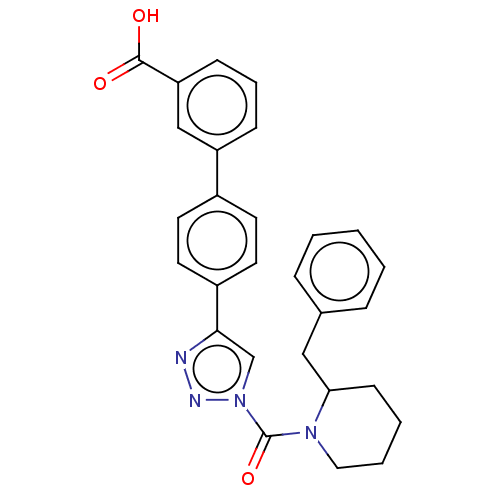 (CHEMBL3263582) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In situ inhibition of ABHD6 in mouse Neuro2a cells after 4 hrs by ABPP-SILAC-based LC-MS/MS analysis | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010336 (CHEMBL3263579) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In situ inhibition of ABHD6 in mouse Neuro2a cells after 4 hrs by gel-based competitive ABPP assay | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010335 (CHEMBL3263577) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In situ inhibition of ABHD6 in mouse Neuro2a cells after 4 hrs by gel-based competitive ABPP assay | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010337 (CHEMBL3263582) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In situ inhibition of ABHD6 in mouse Neuro2a cells after 4 hrs by gel-based competitive ABPP assay | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010337 (CHEMBL3263582) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of ABHD6 in FP-rhodamine-labelled mouse brain membrane preincubated for 30 mins followed by FP-rhodamine labelling measured after 30 mins ... | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010336 (CHEMBL3263579) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of ABHD6 in FP-rhodamine-labelled mouse brain membrane preincubated for 30 mins followed by FP-rhodamine labelling measured after 30 mins ... | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010335 (CHEMBL3263577) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of ABHD6 in FP-rhodamine-labelled mouse brain membrane preincubated for 30 mins followed by FP-rhodamine labelling measured after 30 mins ... | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50549706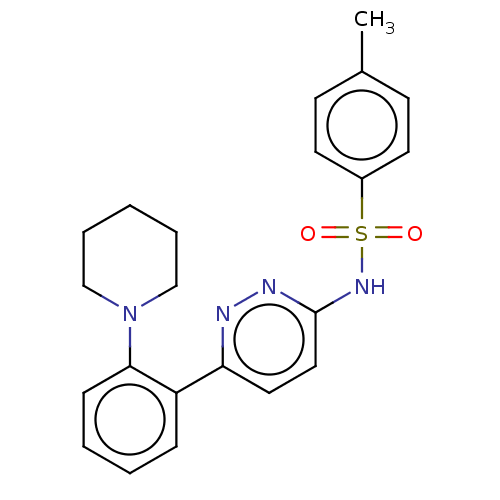 (CHEMBL4744083) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010337 (CHEMBL3263582) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant mouse ABHD6 expressed in HEK293T using 2-arachidonoylglycerol preincubated for 30 mins followed by substrate addition measu... | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576346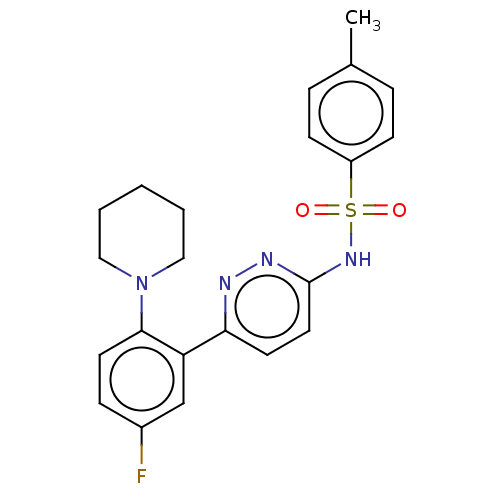 (CHEMBL4858480) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192306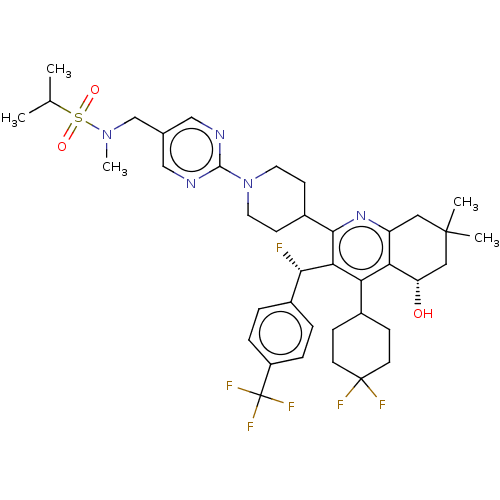 (US9187450, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192305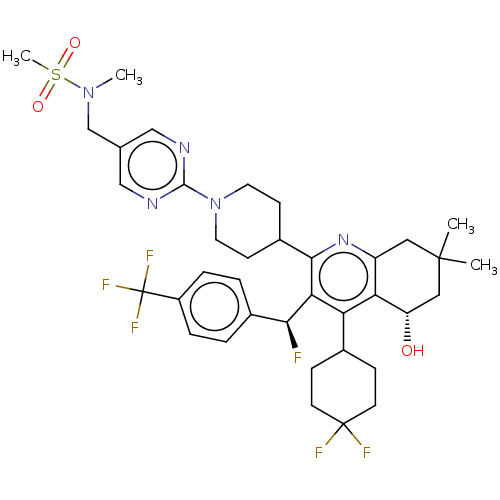 (US9187450, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576347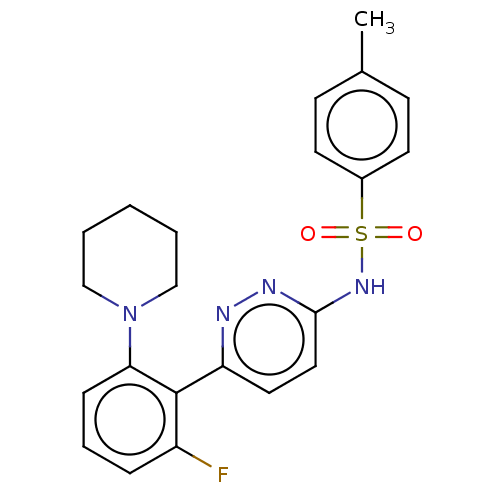 (CHEMBL4849479) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192303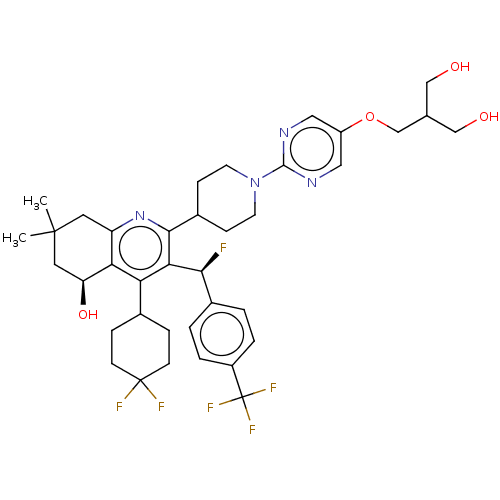 (US9187450, 49 | US9321747, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192295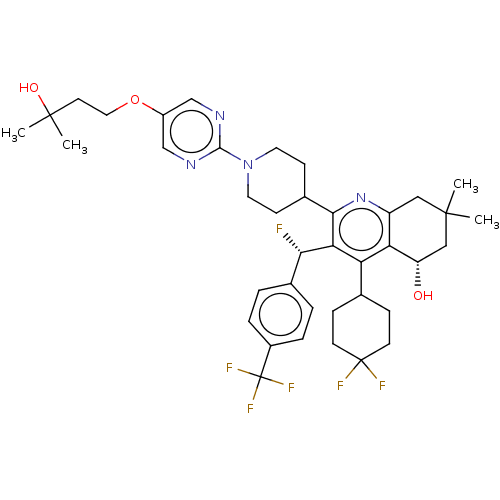 (US9187450, 43 | US9321747, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576345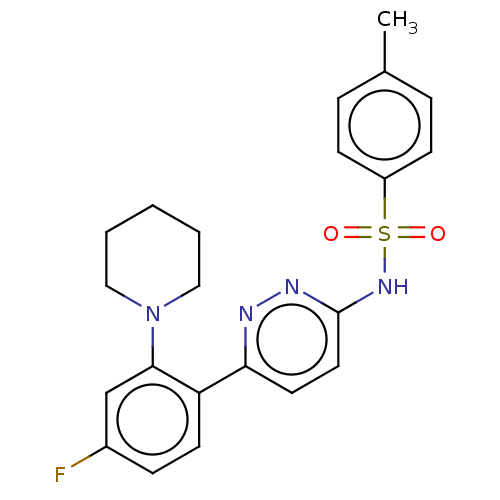 (CHEMBL4853796) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192289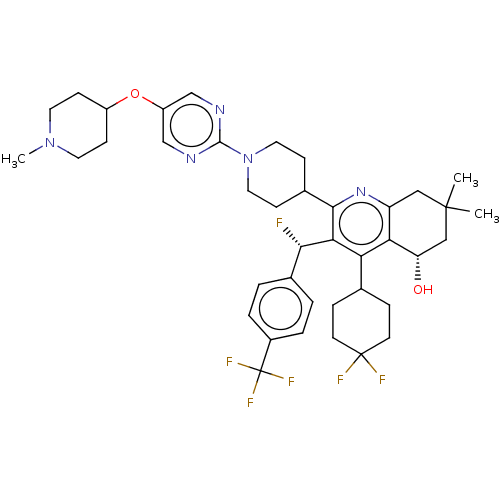 (US9187450, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192292 (US9187450, 40 | US9321747, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192298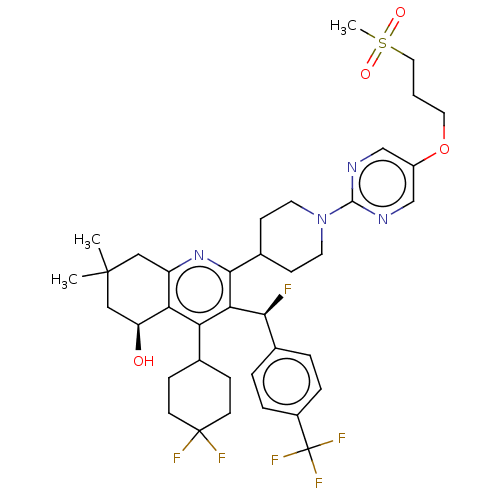 (US9187450, 45 | US9321747, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192304 (US9187450, 50 | US9321747, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576356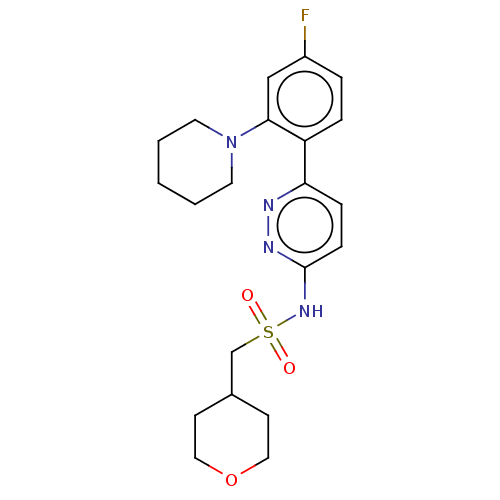 (CHEMBL4854493) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576355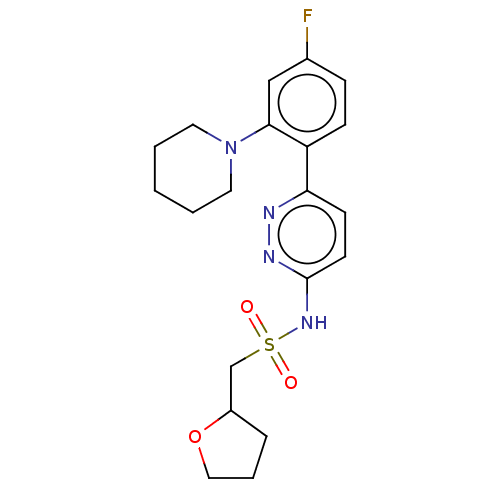 (CHEMBL4872767) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010336 (CHEMBL3263579) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant mouse ABHD6 expressed in HEK293T using 2-arachidonoylglycerol preincubated for 30 mins followed by substrate addition measu... | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Mus musculus (mouse)) | BDBM50010335 (CHEMBL3263577) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant mouse ABHD6 expressed in HEK293T using 2-arachidonoylglycerol preincubated for 30 mins followed by substrate addition measu... | Cell Chem Biol 56: 8270-9 (2013) Article DOI: 10.1021/jm400899c BindingDB Entry DOI: 10.7270/Q2WM1FXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192293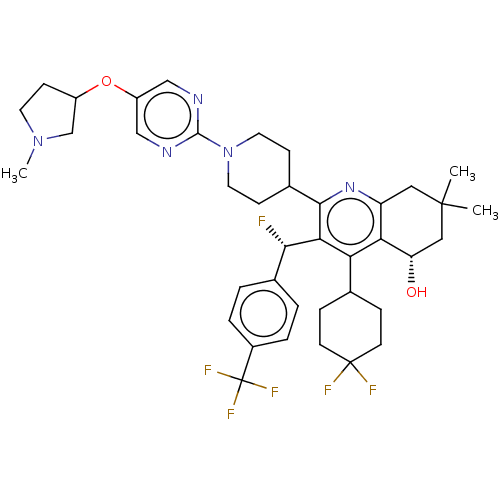 (US9187450, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192299 (US9187450, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576357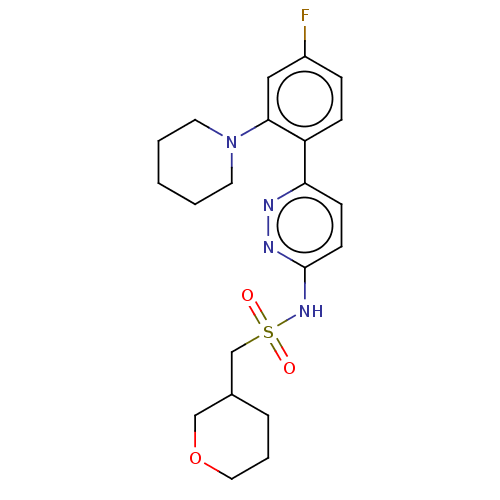 (CHEMBL4850268) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192274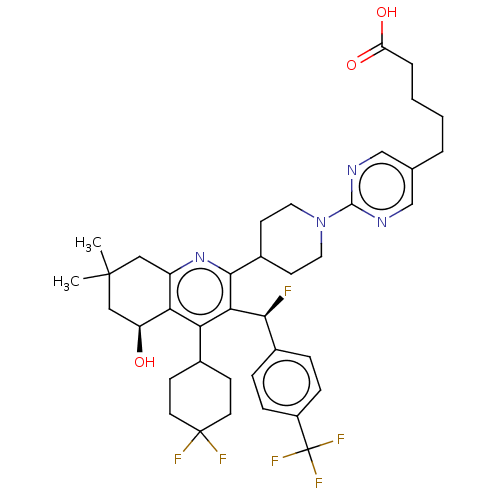 (US9187450, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192254 (US9187450, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192281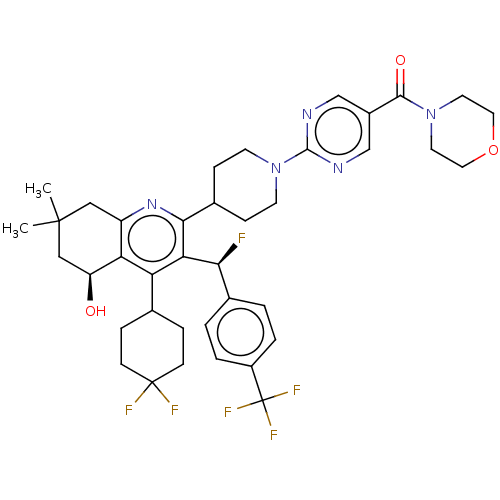 (US9187450, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576344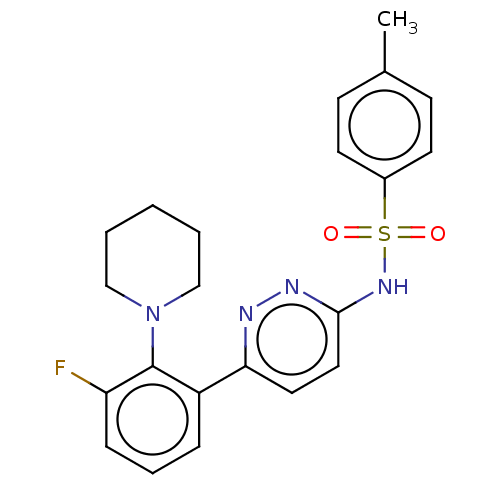 (CHEMBL4851794) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192296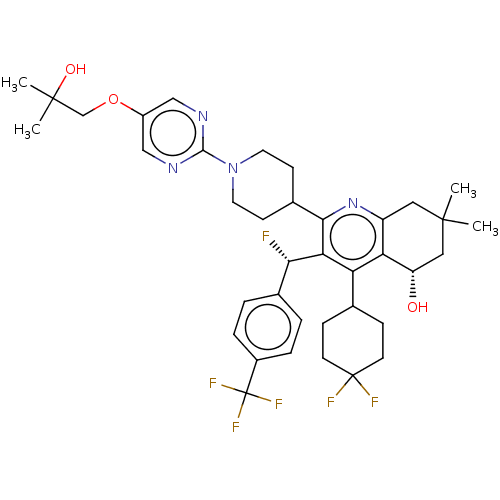 (US9187450, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192294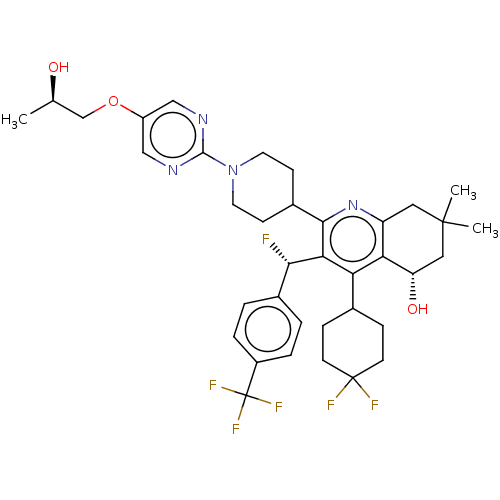 (US9187450, 42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192287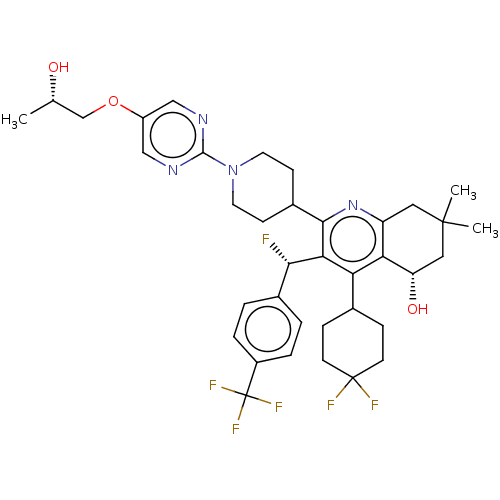 (US9187450, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576350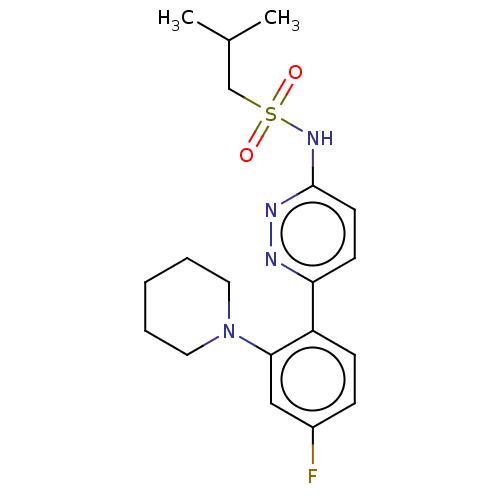 (CHEMBL4877327) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192312 (US9187450, 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576351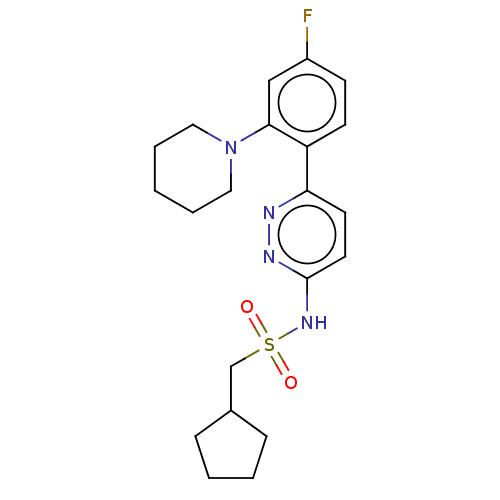 (CHEMBL4866526) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192280 (US9187450, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192301 (US9187450, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192308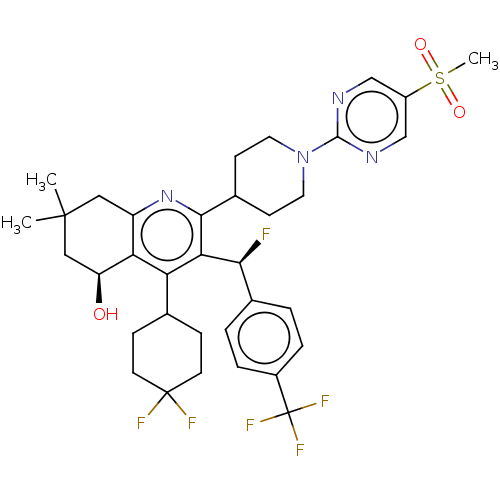 (US9187450, 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192290 (US9187450, 39 | US9321747, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192279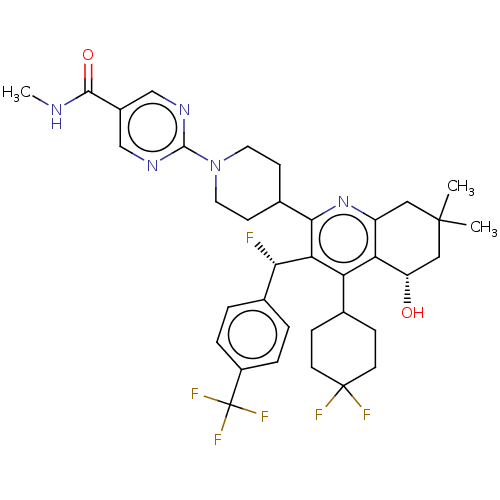 (US9187450, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192315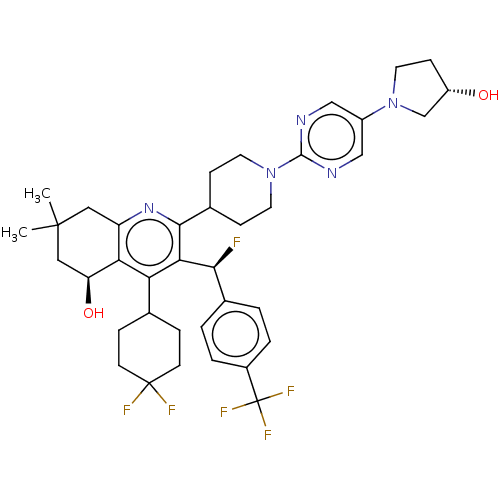 (US9187450, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576358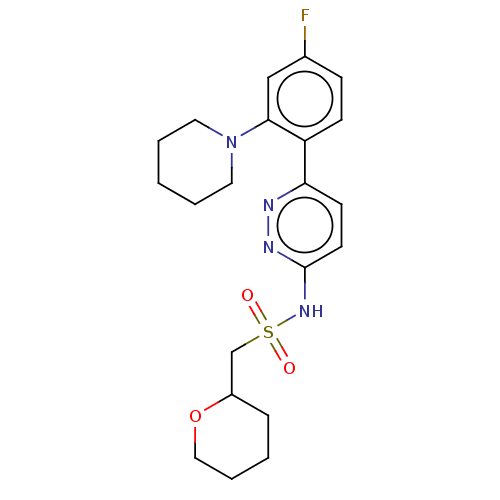 (CHEMBL4869777) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192307 (US9187450, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50072084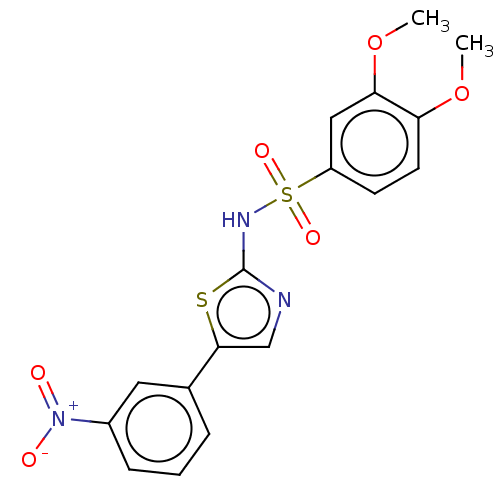 (CHEMBL3407930) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192285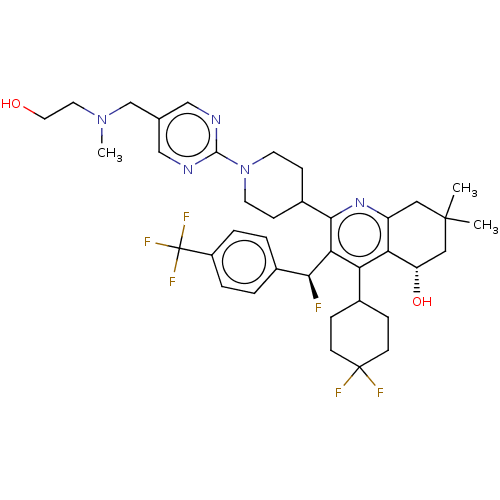 (US9187450, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50576349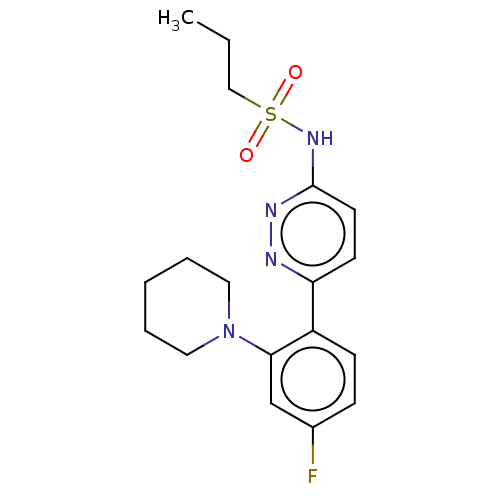 (CHEMBL4873105) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver mitochondrial KMO assessed as reduction in 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 min... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128115 BindingDB Entry DOI: 10.7270/Q2XW4PN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 258 total ) | Next | Last >> |