| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Methionine--tRNA ligase, cytoplasmic |
|---|
| Ligand | BDBM50143343 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_105285 (CHEMBL718952) |
|---|
| IC50 | >100000±n/a nM |
|---|
| Citation |  Tandon, M; Coffen, DL; Gallant, P; Keith, D; Ashwell, MA Potent and selective inhibitors of bacterial methionyl tRNA synthetase derived from an oxazolone-dipeptide scaffold. Bioorg Med Chem Lett14:1909-11 (2004) [PubMed] Article Tandon, M; Coffen, DL; Gallant, P; Keith, D; Ashwell, MA Potent and selective inhibitors of bacterial methionyl tRNA synthetase derived from an oxazolone-dipeptide scaffold. Bioorg Med Chem Lett14:1909-11 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Methionine--tRNA ligase, cytoplasmic |
|---|
| Name: | Methionine--tRNA ligase, cytoplasmic |
|---|
| Synonyms: | MARS | MARS1 | Methionyl-tRNA synthetase | SYMC_HUMAN |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 101110.04 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_105283 |
|---|
| Residue: | 900 |
|---|
| Sequence: | MRLFVSDGVPGCLPVLAAAGRARGRAEVLISTVGPEDCVVPFLTRPKVPVLQLDSGNYLF
STSAICRYFFLLSGWEQDDLTNQWLEWEATELQPALSAALYYLVVQGKKGEDVLGSVRRA
LTHIDHSLSRQNCPFLAGETESLADIVLWGALYPLLQDPAYLPEELSALHSWFQTLSTQE
PCQRAAETVLKQQGVLALRPYLQKQPQPSPAEGRAVTNEPEEEELATLSEEEIAMAVTAW
EKGLESLPPLRPQQNPVLPVAGERNVLITSALPYVNNVPHLGNIIGCVLSADVFARYSRL
RQWNTLYLCGTDEYGTATETKALEEGLTPQEICDKYHIIHADIYRWFNISFDIFGRTTTP
QQTKITQDIFQQLLKRGFVLQDTVEQLRCEHCARFLADRFVEGVCPFCGYEEARGDQCDK
CGKLINAVELKKPQCKVCRSCPVVQSSQHLFLDLPKLEKRLEEWLGRTLPGSDWTPNAQF
ITRSWLRDGLKPRCITRDLKWGTPVPLEGFEDKVFYVWFDATIGYLSITANYTDQWERWW
KNPEQVDLYQFMAKDNVPFHSLVFPCSALGAEDNYTLVSHLIATEYLNYEDGKFSKSRGV
GVFGDMAQDTGIPADIWRFYLLYIRPEGQDSAFSWTDLLLKNNSELLNNLGNFINRAGMF
VSKFFGGYVPEMVLTPDDQRLLAHVTLELQHYHQLLEKVRIRDALRSILTISRHGNQYIQ
VNEPWKRIKGSEADRQRAGTVTGLAVNIAALLSVMLQPYMPTVSATIQAQLQLPPPACSI
LLTNFLCTLPAGHQIGTVSPLFQKLENDQIESLRQRFGGGQAKTSPKPAVVETVTTAKPQ
QIQALMDEVTKQGNIVRELKAQKADKNEVAAEVAKLLDLKKQLAVAEGKPPEAPKGKKKK
|
|
|
|---|
| BDBM50143343 |
|---|
| n/a |
|---|
| Name | BDBM50143343 |
|---|
| Synonyms: | (S)-1-{1-[2-(2,4-Dichloro-phenyl)-5-oxo-oxazol-(4Z)-ylidene]-ethyl}-pyrrolidine-2-carboxylic acid [(S)-1-carbamoyl-2-(4-hydroxy-phenyl)-ethyl]-amide | CHEMBL293288 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H24Cl2N4O5 |
|---|
| Mol. Mass. | 531.388 |
|---|
| SMILES | C\C(N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)=C1\N=C(OC1=O)c1ccc(Cl)cc1Cl |c:25| |
|---|
| Structure | 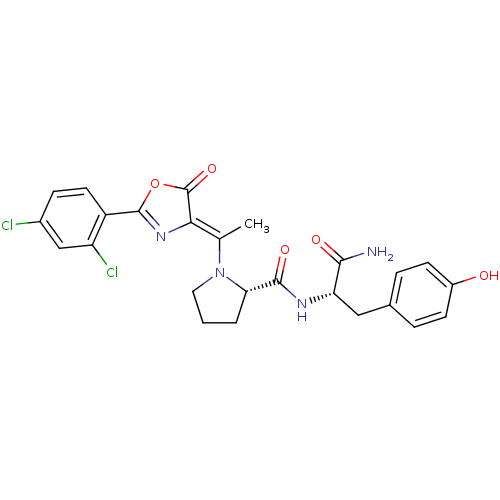
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tandon, M; Coffen, DL; Gallant, P; Keith, D; Ashwell, MA Potent and selective inhibitors of bacterial methionyl tRNA synthetase derived from an oxazolone-dipeptide scaffold. Bioorg Med Chem Lett14:1909-11 (2004) [PubMed] Article
Tandon, M; Coffen, DL; Gallant, P; Keith, D; Ashwell, MA Potent and selective inhibitors of bacterial methionyl tRNA synthetase derived from an oxazolone-dipeptide scaffold. Bioorg Med Chem Lett14:1909-11 (2004) [PubMed] Article