| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, endothelial |
|---|
| Ligand | BDBM50148164 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_65303 (CHEMBL678077) |
|---|
| IC50 | 41000±n/a nM |
|---|
| Citation |  Connolly, S; Aberg, A; Arvai, A; Beaton, HG; Cheshire, DR; Cook, AR; Cooper, S; Cox, D; Hamley, P; Mallinder, P; Millichip, I; Nicholls, DJ; Rosenfeld, RJ; St-Gallay, SA; Tainer, J; Tinker, AC; Wallace, AV 2-aminopyridines as highly selective inducible nitric oxide synthase inhibitors. Differential binding modes dependent on nitrogen substitution. J Med Chem47:3320-3 (2004) [PubMed] Article Connolly, S; Aberg, A; Arvai, A; Beaton, HG; Cheshire, DR; Cook, AR; Cooper, S; Cox, D; Hamley, P; Mallinder, P; Millichip, I; Nicholls, DJ; Rosenfeld, RJ; St-Gallay, SA; Tainer, J; Tinker, AC; Wallace, AV 2-aminopyridines as highly selective inducible nitric oxide synthase inhibitors. Differential binding modes dependent on nitrogen substitution. J Med Chem47:3320-3 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nitric oxide synthase, endothelial |
|---|
| Name: | Nitric oxide synthase, endothelial |
|---|
| Synonyms: | Constitutive NOS | EC-NOS | Endothelial NOS | Endothelial nitric oxide synthase | NOS type III | NOS3 | NOS3_HUMAN | NOSIII | Nitric oxide synthase (inducible and endothelial) | Nitric oxide synthase, endothelial (eNOS) | Nitric-oxide synthase (endothelial and brain) | cNOS | eNOS |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 133297.84 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P29474 |
|---|
| Residue: | 1203 |
|---|
| Sequence: | MGNLKSVAQEPGPPCGLGLGLGLGLCGKQGPATPAPEPSRAPASLLPPAPEHSPPSSPLT
QPPEGPKFPRVKNWEVGSITYDTLSAQAQQDGPCTPRRCLGSLVFPRKLQGRPSPGPPAP
EQLLSQARDFINQYYSSIKRSGSQAHEQRLQEVEAEVAATGTYQLRESELVFGAKQAWRN
APRCVGRIQWGKLQVFDARDCRSAQEMFTYICNHIKYATNRGNLRSAITVFPQRCPGRGD
FRIWNSQLVRYAGYRQQDGSVRGDPANVEITELCIQHGWTPGNGRFDVLPLLLQAPDDPP
ELFLLPPELVLEVPLEHPTLEWFAALGLRWYALPAVSNMLLEIGGLEFPAAPFSGWYMST
EIGTRNLCDPHRYNILEDVAVCMDLDTRTTSSLWKDKAAVEINVAVLHSYQLAKVTIVDH
HAATASFMKHLENEQKARGGCPADWAWIVPPISGSLTPVFHQEMVNYFLSPAFRYQPDPW
KGSAAKGTGITRKKTFKEVANAVKISASLMGTVMAKRVKATILYGSETGRAQSYAQQLGR
LFRKAFDPRVLCMDEYDVVSLEHETLVLVVTSTFGNGDPPENGESFAAALMEMSGPYNSS
PRPEQHKSYKIRFNSISCSDPLVSSWRRKRKESSNTDSAGALGTLRFCVFGLGSRAYPHF
CAFARAVDTRLEELGGERLLQLGQGDELCGQEEAFRGWAQAAFQAACETFCVGEDAKAAA
RDIFSPKRSWKRQRYRLSAQAEGLQLLPGLIHVHRRKMFQATIRSVENLQSSKSTRATIL
VRLDTGGQEGLQYQPGDHIGVCPPNRPGLVEALLSRVEDPPAPTEPVAVEQLEKGSPGGP
PPGWVRDPRLPPCTLRQALTFFLDITSPPSPQLLRLLSTLAEEPREQQELEALSQDPRRY
EEWKWFRCPTLLEVLEQFPSVALPAPLLLTQLPLLQPRYYSVSSAPSTHPGEIHLTVAVL
AYRTQDGLGPLHYGVCSTWLSQLKPGDPVPCFIRGAPSFRLPPDPSLPCILVGPGTGIAP
FRGFWQERLHDIESKGLQPTPMTLVFGCRCSQLDHLYRDEVQNAQQRGVFGRVLTAFSRE
PDNPKTYVQDILRTELAAEVHRVLCLERGHMFVCGDVTMATNVLQTVQRILATEGDMELD
EAGDVIGVLRDQQRYHEDIFGLTLRTQEVTSRIRTQSFSLQERQLRGAVPWAFDPPGSDT
NSP
|
|
|
|---|
| BDBM50148164 |
|---|
| n/a |
|---|
| Name | BDBM50148164 |
|---|
| Synonyms: | 4-(4-Methoxy-pyridin-2-ylamino)-piperidine-1-carboxylic acid ethyl ester | CHEMBL112246 | Ethyl 4-[(4-methoxypyridin-2-yl)amino]piperidine-1-carboxylate, 11 | ethyl 4-(4-methoxypyridin-2-ylamino)piperidine-1-carboxylate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H21N3O3 |
|---|
| Mol. Mass. | 279.3348 |
|---|
| SMILES | CCOC(=O)N1CCC(CC1)Nc1cc(OC)ccn1 |
|---|
| Structure | 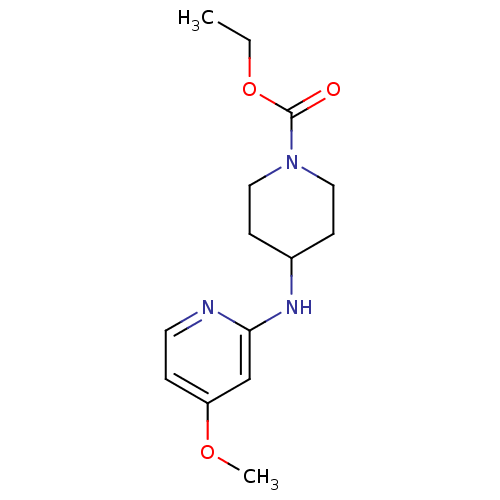
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Connolly, S; Aberg, A; Arvai, A; Beaton, HG; Cheshire, DR; Cook, AR; Cooper, S; Cox, D; Hamley, P; Mallinder, P; Millichip, I; Nicholls, DJ; Rosenfeld, RJ; St-Gallay, SA; Tainer, J; Tinker, AC; Wallace, AV 2-aminopyridines as highly selective inducible nitric oxide synthase inhibitors. Differential binding modes dependent on nitrogen substitution. J Med Chem47:3320-3 (2004) [PubMed] Article
Connolly, S; Aberg, A; Arvai, A; Beaton, HG; Cheshire, DR; Cook, AR; Cooper, S; Cox, D; Hamley, P; Mallinder, P; Millichip, I; Nicholls, DJ; Rosenfeld, RJ; St-Gallay, SA; Tainer, J; Tinker, AC; Wallace, AV 2-aminopyridines as highly selective inducible nitric oxide synthase inhibitors. Differential binding modes dependent on nitrogen substitution. J Med Chem47:3320-3 (2004) [PubMed] Article