

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | GTPase KRas | ||
| Ligand | BDBM50590848 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_2198434 (CHEMBL5110950) | ||
| Kd | 97±n/a nM | ||
| Citation |  Li, L; Zhao, H; Peng, X; Liu, J; Mai, R; Chen, J; Lin, L; Chen, T; Yan, J; Shi, J; Chen, J Discovery of novel Quinazoline-based KRAS G12C inhibitors as potential anticancer agents. Bioorg Med Chem71:0 (2022) [PubMed] Article Li, L; Zhao, H; Peng, X; Liu, J; Mai, R; Chen, J; Lin, L; Chen, T; Yan, J; Shi, J; Chen, J Discovery of novel Quinazoline-based KRAS G12C inhibitors as potential anticancer agents. Bioorg Med Chem71:0 (2022) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| GTPase KRas | |||
| Name: | GTPase KRas | ||
| Synonyms: | GTPase KRas, N-terminally processed | K-Ras 2 | KRAS | KRAS2 | Ki-Ras | RASK2 | RASK_HUMAN | c-K-ras | c-Ki-ras | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 21656.10 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | ChEMBL_1476955 | ||
| Residue: | 189 | ||
| Sequence: |
| ||
| BDBM50590848 | |||
| n/a | |||
| Name | BDBM50590848 | ||
| Synonyms: | CHEMBL5191782 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C24H18Cl2FN5O5 | ||
| Mol. Mass. | 546.335 | ||
| SMILES | COc1ccc(Cl)cc1-c1c(Cl)cc2c(ncnc2c1F)N1CCN(CC1)C(=O)c1ccc(o1)[N+]([O-])=O |(-6.36,-2.29,;-5.03,-3.06,;-5.03,-4.6,;-6.36,-5.38,;-6.35,-6.91,;-5.02,-7.68,;-5.02,-9.22,;-3.69,-6.92,;-3.69,-5.38,;-2.35,-4.61,;-2.35,-3.06,;-3.69,-2.29,;-1.02,-2.29,;.31,-3.06,;1.64,-2.29,;2.97,-3.06,;2.98,-4.59,;1.65,-5.37,;.31,-4.6,;-1.02,-5.37,;-1.02,-6.91,;1.64,-.75,;.31,.02,;.31,1.56,;1.64,2.33,;2.97,1.56,;2.97,.02,;1.64,3.87,;.31,4.64,;2.97,4.64,;4.43,4.18,;5.35,5.43,;4.47,6.65,;2.97,6.18,;4.87,8.13,;6.36,8.53,;3.78,9.22,)| | ||
| Structure | 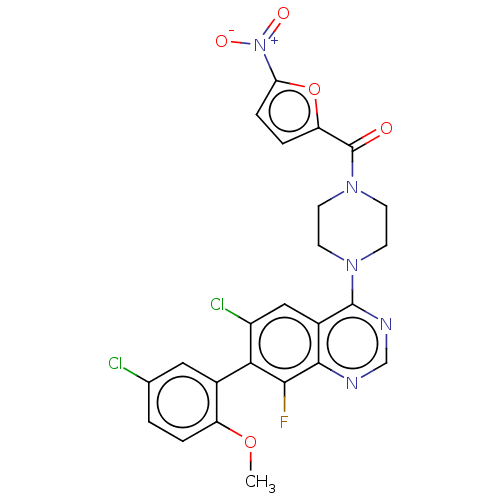
| ||