 Found 17379 hits with Last Name = 'yan' and Initial = 'j'
Found 17379 hits with Last Name = 'yan' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207217
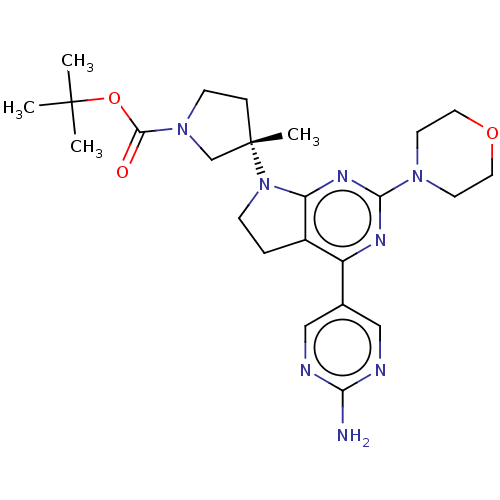
(US9260439, 194 | US9260439, 238 | US9260439, 239)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C24H34N8O3/c1-23(2,3)35-22(33)31-8-6-24(4,15-31)32-7-5-17-18(16-13-26-20(25)27-14-16)28-21(29-19(17)32)30-9-11-34-12-10-30/h13-14H,5-12,15H2,1-4H3,(H2,25,26,27)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340314
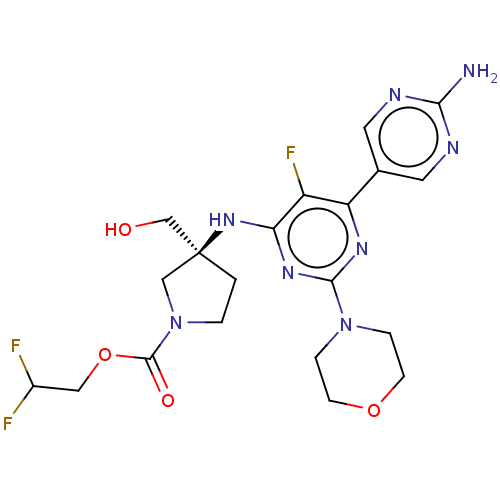
((Scheme A): Preparation of 2,2-difluoroethyl (3S)-...)Show SMILES Nc1ncc(cn1)-c1nc(nc(N[C@@]2(CO)CCN(C2)C(=O)OCC(F)F)c1F)N1CCOCC1 |r| Show InChI InChI=1S/C20H25F3N8O4/c21-13(22)9-35-19(33)31-2-1-20(10-31,11-32)29-16-14(23)15(12-7-25-17(24)26-8-12)27-18(28-16)30-3-5-34-6-4-30/h7-8,13,32H,1-6,9-11H2,(H2,24,25,26)(H,27,28,29)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340384
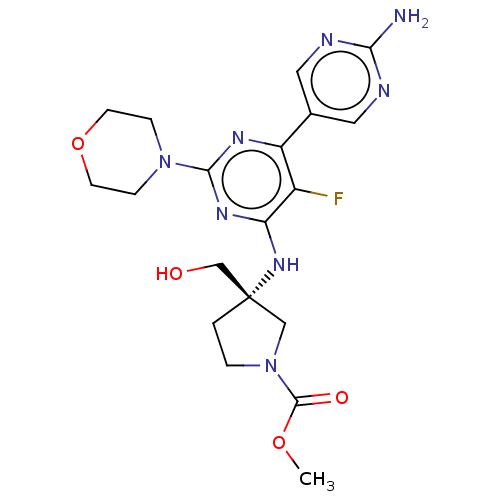
(US9758538, Example 72)Show SMILES COC(=O)N1CC[C@@](CO)(C1)Nc1nc(nc(c1F)-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C19H25FN8O4/c1-31-18(30)28-3-2-19(10-28,11-29)26-15-13(20)14(12-8-22-16(21)23-9-12)24-17(25-15)27-4-6-32-7-5-27/h8-9,29H,2-7,10-11H2,1H3,(H2,21,22,23)(H,24,25,26)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207196
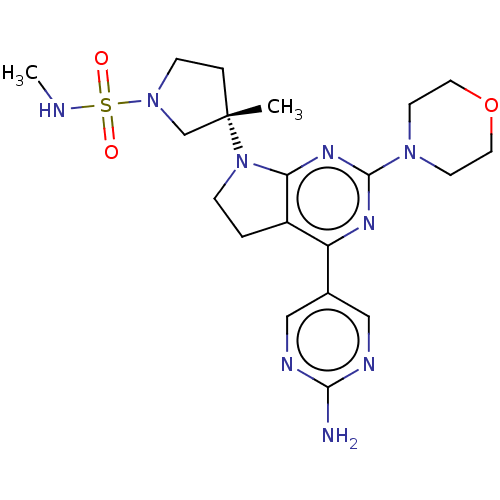
(US9260439, 173)Show SMILES CNS(=O)(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C20H29N9O3S/c1-20(4-6-28(13-20)33(30,31)22-2)29-5-3-15-16(14-11-23-18(21)24-12-14)25-19(26-17(15)29)27-7-9-32-10-8-27/h11-12,22H,3-10,13H2,1-2H3,(H2,21,23,24)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207378
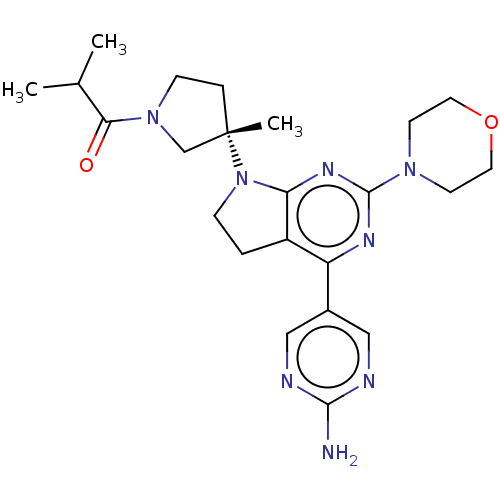
(US9260439, 262)Show SMILES CC(C)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C23H32N8O2/c1-15(2)20(32)30-7-5-23(3,14-30)31-6-4-17-18(16-12-25-21(24)26-13-16)27-22(28-19(17)31)29-8-10-33-11-9-29/h12-13,15H,4-11,14H2,1-3H3,(H2,24,25,26)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340336
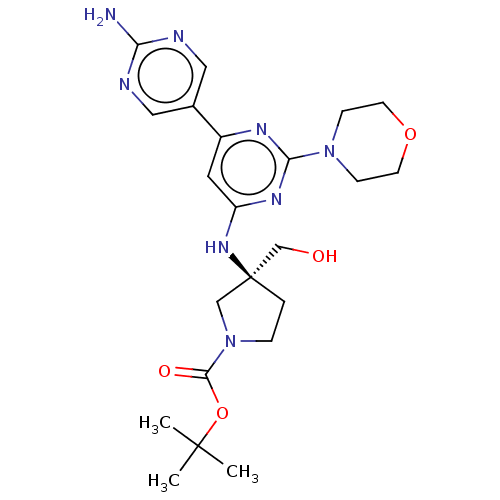
(US9758538, Example 24)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C22H32N8O4/c1-21(2,3)34-20(32)30-5-4-22(13-30,14-31)28-17-10-16(15-11-24-18(23)25-12-15)26-19(27-17)29-6-8-33-9-7-29/h10-12,31H,4-9,13-14H2,1-3H3,(H2,23,24,25)(H,26,27,28)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340346

(US9758538, Example 34)Show SMILES COC(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C19H26N8O4/c1-30-18(29)27-3-2-19(11-27,12-28)25-15-8-14(13-9-21-16(20)22-10-13)23-17(24-15)26-4-6-31-7-5-26/h8-10,28H,2-7,11-12H2,1H3,(H2,20,21,22)(H,23,24,25)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100528
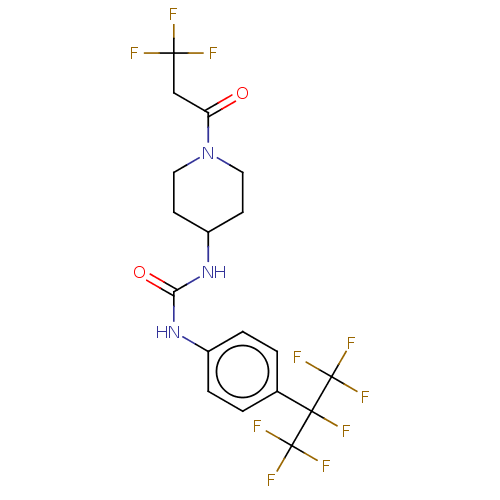
(CHEMBL3327081)Show SMILES FC(F)(F)CC(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H17F10N3O2/c19-15(20,21)9-13(32)31-7-5-12(6-8-31)30-14(33)29-11-3-1-10(2-4-11)16(22,17(23,24)25)18(26,27)28/h1-4,12H,5-9H2,(H2,29,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100535
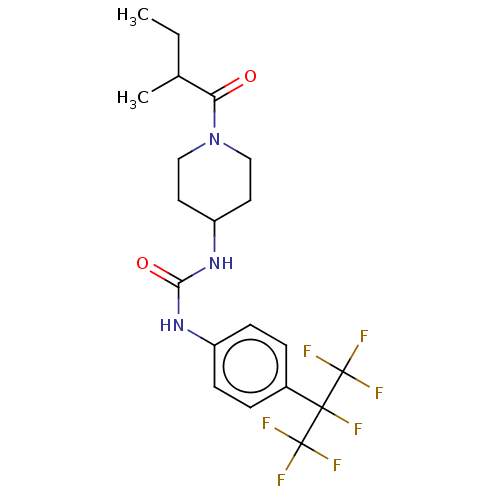
(CHEMBL3327073)Show SMILES CCC(C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H24F7N3O2/c1-3-12(2)16(31)30-10-8-15(9-11-30)29-17(32)28-14-6-4-13(5-7-14)18(21,19(22,23)24)20(25,26)27/h4-7,12,15H,3,8-11H2,1-2H3,(H2,28,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340391
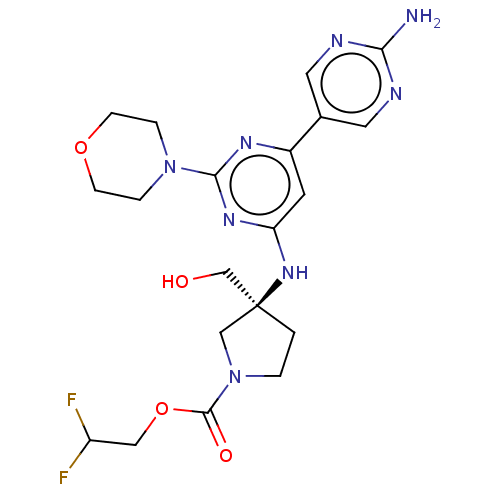
(US9758538, Example 79)Show SMILES Nc1ncc(cn1)-c1cc(N[C@@]2(CO)CCN(C2)C(=O)OCC(F)F)nc(n1)N1CCOCC1 |r| Show InChI InChI=1S/C20H26F2N8O4/c21-15(22)10-34-19(32)30-2-1-20(11-30,12-31)28-16-7-14(13-8-24-17(23)25-9-13)26-18(27-16)29-3-5-33-6-4-29/h7-9,15,31H,1-6,10-12H2,(H2,23,24,25)(H,26,27,28)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207236
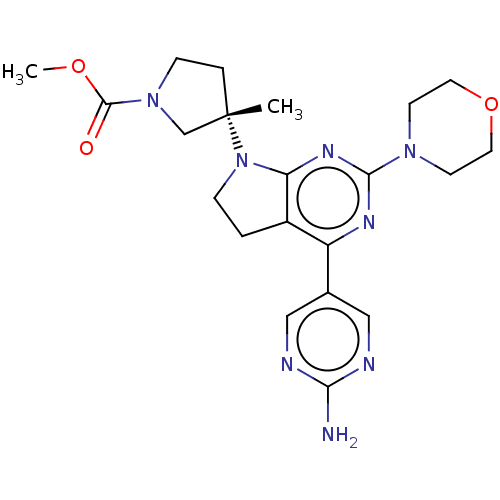
(US9260439, 213)Show SMILES COC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H28N8O3/c1-21(4-6-28(13-21)20(30)31-2)29-5-3-15-16(14-11-23-18(22)24-12-14)25-19(26-17(15)29)27-7-9-32-10-8-27/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207028
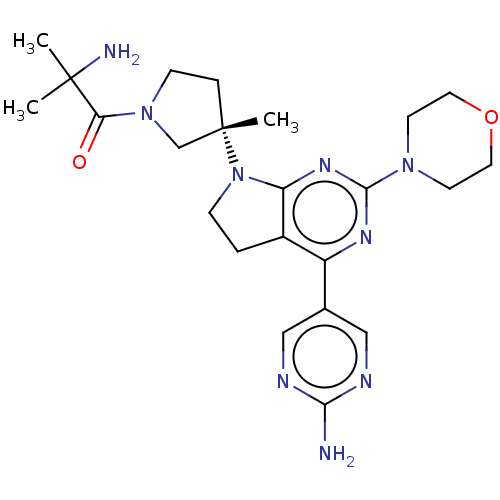
(US9260439, 10 | US9260439, 4)Show SMILES CC(C)(N)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C23H33N9O2/c1-22(2,25)19(33)31-7-5-23(3,14-31)32-6-4-16-17(15-12-26-20(24)27-13-15)28-21(29-18(16)32)30-8-10-34-11-9-30/h12-13H,4-11,14,25H2,1-3H3,(H2,24,26,27)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207172
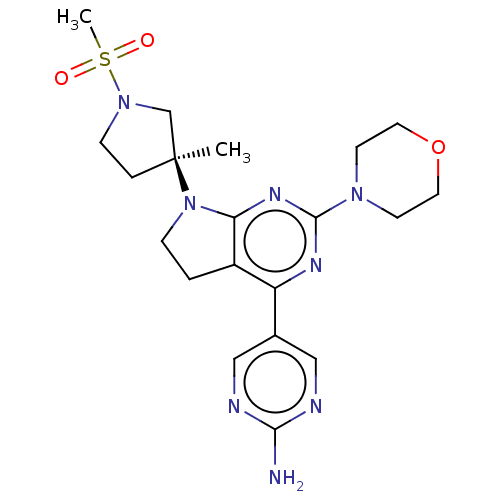
(US9260439, 149)Show SMILES C[C@@]1(CCN(C1)S(C)(=O)=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C20H28N8O3S/c1-20(4-6-27(13-20)32(2,29)30)28-5-3-15-16(14-11-22-18(21)23-12-14)24-19(25-17(15)28)26-7-9-31-10-8-26/h11-12H,3-10,13H2,1-2H3,(H2,21,22,23)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207391
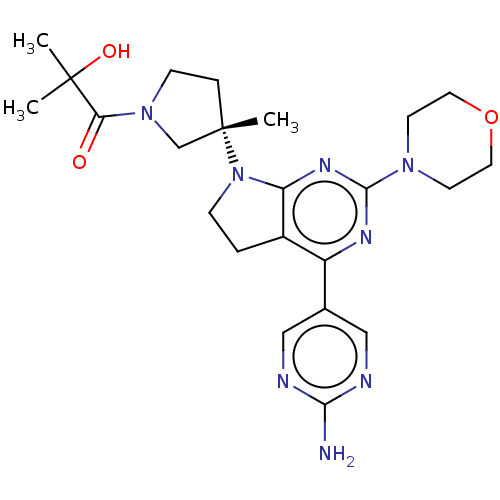
(US9260439, 275)Show SMILES CC(C)(O)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C23H32N8O3/c1-22(2,33)19(32)30-7-5-23(3,14-30)31-6-4-16-17(15-12-25-20(24)26-13-15)27-21(28-18(16)31)29-8-10-34-11-9-29/h12-13,33H,4-11,14H2,1-3H3,(H2,24,25,26)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM21865
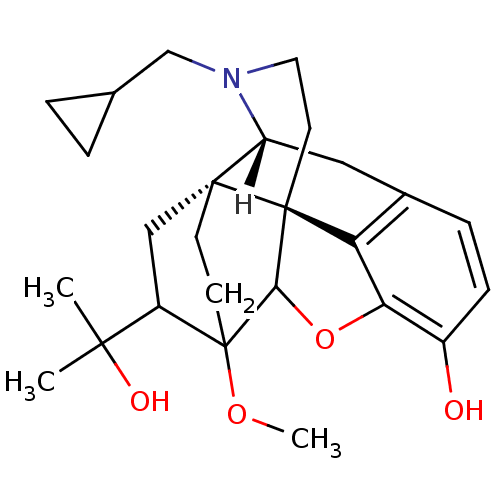
((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...)Show SMILES [H][C@]12Cc3ccc(O)c4OC5[C@](CCN1CC1CC1)(c34)[C@@]21CCC5(OC)C(C1)C(C)(C)O |TLB:28:26:11.10:21.22,8:19:20:14.12.13,4:3:20:14.12.13| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18?,19-,22?,24-,25+,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005
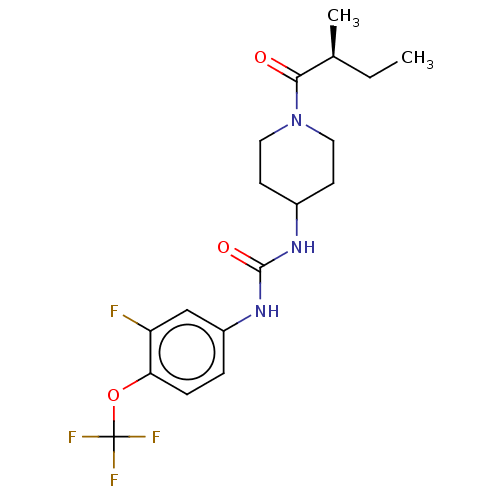
(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM517699
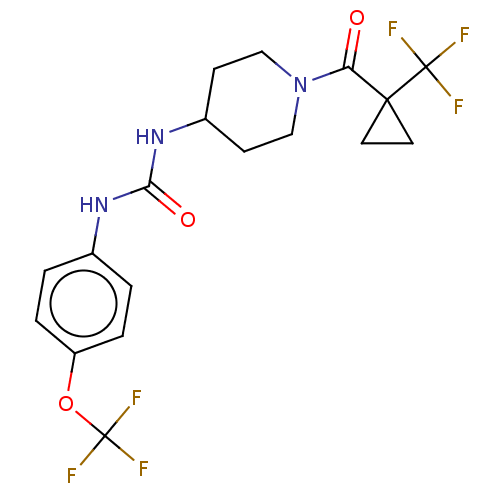
(US11123311, Compound 28)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)C2(CC2)C(F)(F)F)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409009
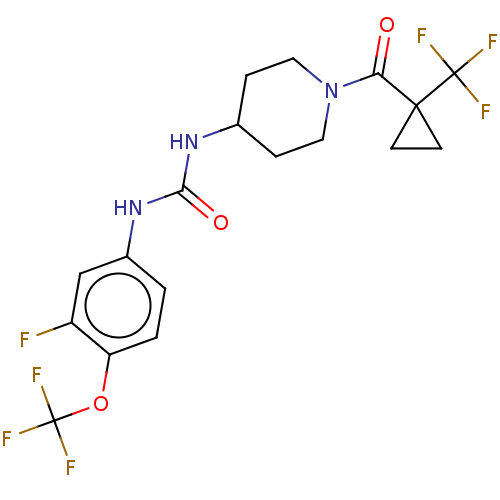
(US10377744, Compound No. 30 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2(CC2)C(F)(F)F)ccc1OC(F)(F)F Show InChI InChI=1S/C18H18F7N3O3/c19-12-9-11(1-2-13(12)31-18(23,24)25)27-15(30)26-10-3-7-28(8-4-10)14(29)16(5-6-16)17(20,21)22/h1-2,9-10H,3-8H2,(H2,26,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005

(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005

(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115735
BindingDB Entry DOI: 10.7270/Q2SF30T4 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409009

(US10377744, Compound No. 30 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2(CC2)C(F)(F)F)ccc1OC(F)(F)F Show InChI InChI=1S/C18H18F7N3O3/c19-12-9-11(1-2-13(12)31-18(23,24)25)27-15(30)26-10-3-7-28(8-4-10)14(29)16(5-6-16)17(20,21)22/h1-2,9-10H,3-8H2,(H2,26,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115735
BindingDB Entry DOI: 10.7270/Q2SF30T4 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409020
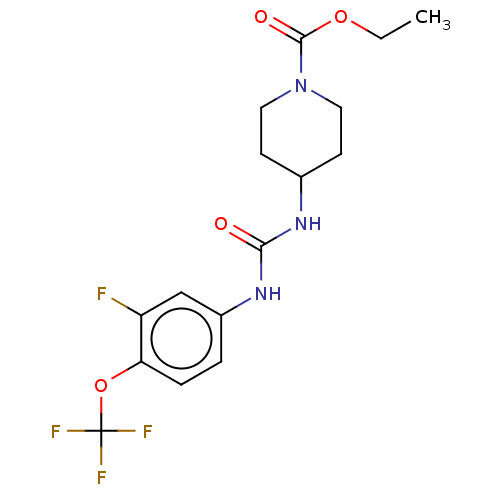
(US10377744, Compound No. 40 | US11123311, Compound...)Show SMILES CCOC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 Show InChI InChI=1S/C16H19F4N3O4/c1-2-26-15(25)23-7-5-10(6-8-23)21-14(24)22-11-3-4-13(12(17)9-11)27-16(18,19)20/h3-4,9-10H,2,5-8H2,1H3,(H2,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115735
BindingDB Entry DOI: 10.7270/Q2SF30T4 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409020

(US10377744, Compound No. 40 | US11123311, Compound...)Show SMILES CCOC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 Show InChI InChI=1S/C16H19F4N3O4/c1-2-26-15(25)23-7-5-10(6-8-23)21-14(24)22-11-3-4-13(12(17)9-11)27-16(18,19)20/h3-4,9-10H,2,5-8H2,1H3,(H2,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409030
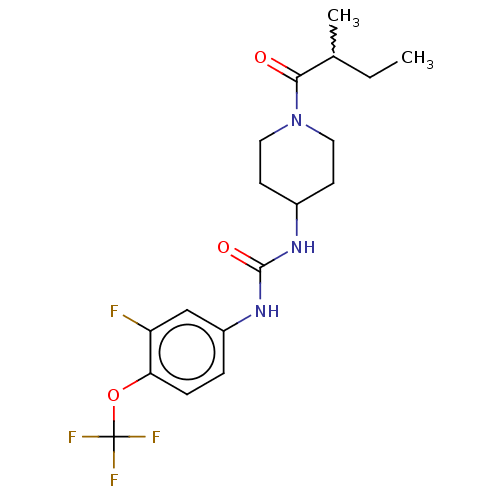
(US10377744, Compound No. 51 | US11123311, Compound...)Show SMILES CCC(C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |w:2.2| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207234
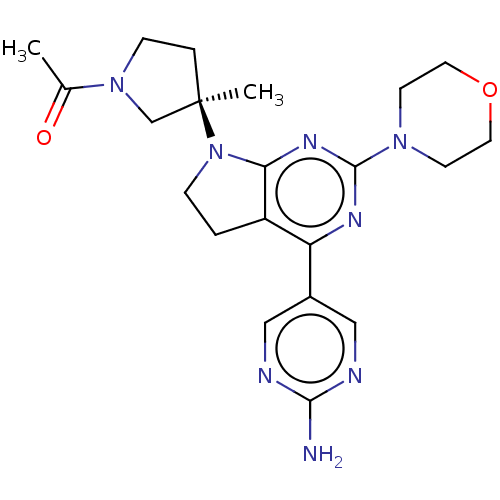
(US9260439, 211)Show SMILES CC(=O)N1CC[C@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H28N8O2/c1-14(30)28-6-4-21(2,13-28)29-5-3-16-17(15-11-23-19(22)24-12-15)25-20(26-18(16)29)27-7-9-31-10-8-27/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409016
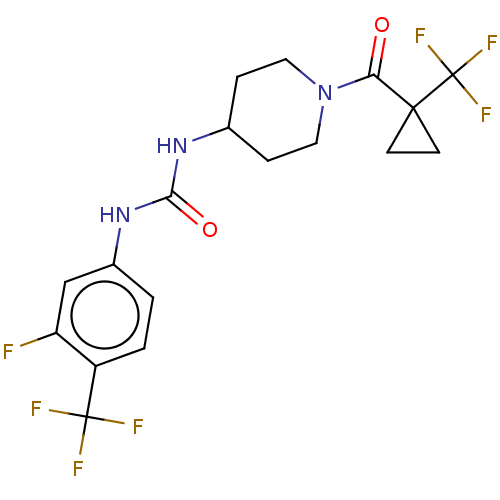
(US10377744, Compound No. 36 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2(CC2)C(F)(F)F)ccc1C(F)(F)F Show InChI InChI=1S/C18H18F7N3O2/c19-13-9-11(1-2-12(13)17(20,21)22)27-15(30)26-10-3-7-28(8-4-10)14(29)16(5-6-16)18(23,24)25/h1-2,9-10H,3-8H2,(H2,26,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409016

(US10377744, Compound No. 36 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2(CC2)C(F)(F)F)ccc1C(F)(F)F Show InChI InChI=1S/C18H18F7N3O2/c19-13-9-11(1-2-12(13)17(20,21)22)27-15(30)26-10-3-7-28(8-4-10)14(29)16(5-6-16)18(23,24)25/h1-2,9-10H,3-8H2,(H2,26,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115735
BindingDB Entry DOI: 10.7270/Q2SF30T4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207239
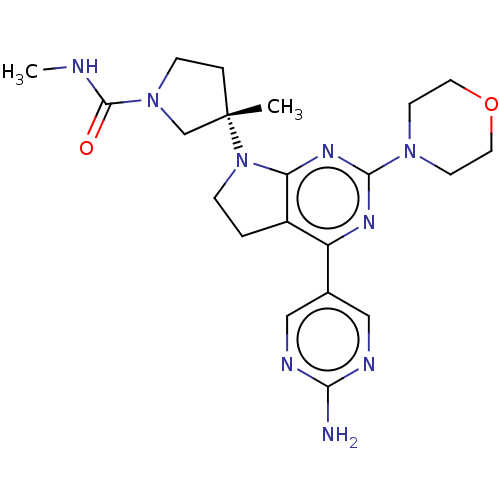
(US9260439, 216)Show SMILES CNC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H29N9O2/c1-21(4-6-29(13-21)20(31)23-2)30-5-3-15-16(14-11-24-18(22)25-12-14)26-19(27-17(15)30)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H,23,31)(H2,22,24,25)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484748
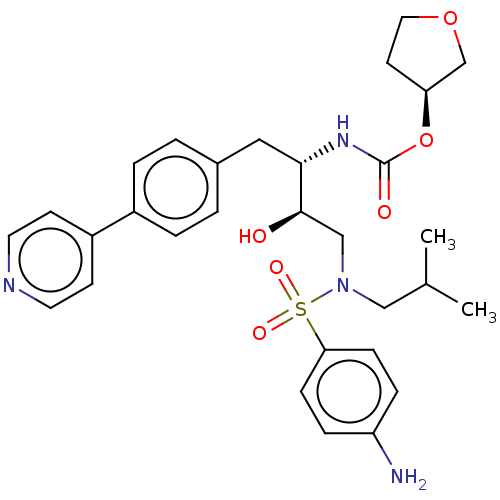
(CHEMBL1957077)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccc(cc1)-c1ccncc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C30H38N4O6S/c1-21(2)18-34(41(37,38)27-9-7-25(31)8-10-27)19-29(35)28(33-30(36)40-26-13-16-39-20-26)17-22-3-5-23(6-4-22)24-11-14-32-15-12-24/h3-12,14-15,21,26,28-29,35H,13,16-20,31H2,1-2H3,(H,33,36)/t26-,28-,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis |
Bioorg Med Chem Lett 22: 1976-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.037
BindingDB Entry DOI: 10.7270/Q2V69ND3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340352
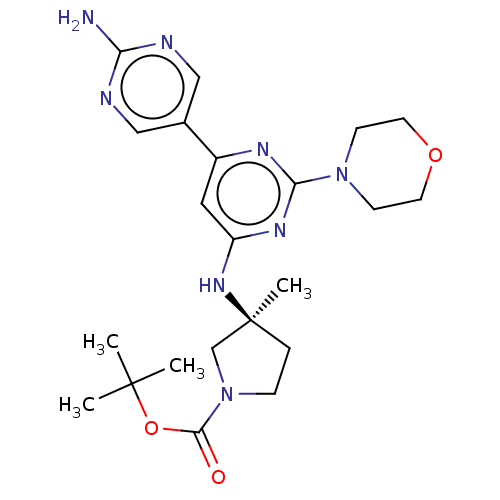
(US9758538, Example 40)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](C)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C22H32N8O3/c1-21(2,3)33-20(31)30-6-5-22(4,14-30)28-17-11-16(15-12-24-18(23)25-13-15)26-19(27-17)29-7-9-32-10-8-29/h11-13H,5-10,14H2,1-4H3,(H2,23,24,25)(H,26,27,28)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50434787
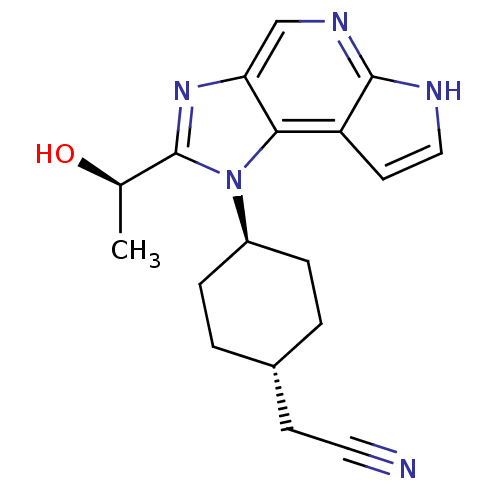
(CHEMBL2386635 | US10487083, Example C | US10703751...)Show SMILES C[C@@H](O)c1nc2cnc3[nH]ccc3c2n1[C@H]1CC[C@H](CC#N)CC1 |r,wU:18.21,wD:15.17,1.1,(49.07,-5.95,;48.31,-7.29,;46.77,-7.3,;49.09,-8.62,;48.48,-10.02,;49.62,-11.04,;49.62,-12.58,;50.95,-13.35,;52.28,-12.58,;53.75,-13.05,;54.65,-11.81,;53.75,-10.56,;52.28,-11.04,;50.94,-10.26,;50.61,-8.77,;51.68,-7.66,;53.17,-8.05,;54.24,-6.95,;53.83,-5.47,;54.9,-4.37,;56.39,-4.75,;57.88,-5.12,;52.34,-5.09,;51.26,-6.19,)| Show InChI InChI=1S/C18H21N5O/c1-11(24)18-22-15-10-21-17-14(7-9-20-17)16(15)23(18)13-4-2-12(3-5-13)6-8-19/h7,9-13,24H,2-6H2,1H3,(H,20,21)/t11-,12-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK1 (unknown origin) using Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 mins in presence of ATP |
J Med Chem 56: 4764-85 (2013)
Article DOI: 10.1021/jm4004895
BindingDB Entry DOI: 10.7270/Q2CV4K40 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246967
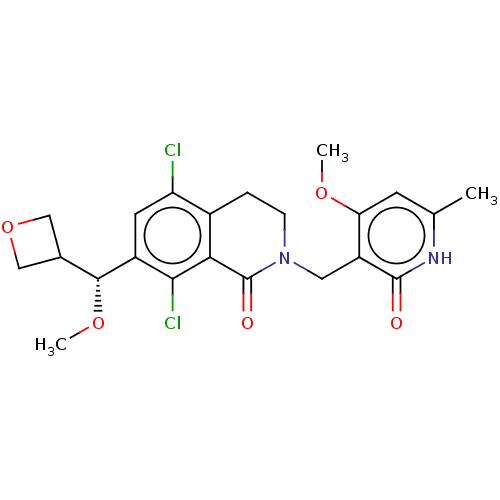
(CHEMBL4080228 | US10570121, Example 81)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C22H24Cl2N2O5/c1-11-6-17(29-2)15(21(27)25-11)8-26-5-4-13-16(23)7-14(19(24)18(13)22(26)28)20(30-3)12-9-31-10-12/h6-7,12,20H,4-5,8-10H2,1-3H3,(H,25,27)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Binding affinity to EZH2 (unknown origin) |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(MOUSE) | BDBM21865

((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...)Show SMILES [H][C@]12Cc3ccc(O)c4OC5[C@](CCN1CC1CC1)(c34)[C@@]21CCC5(OC)C(C1)C(C)(C)O |TLB:28:26:11.10:21.22,8:19:20:14.12.13,4:3:20:14.12.13| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18?,19-,22?,24-,25+,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207061
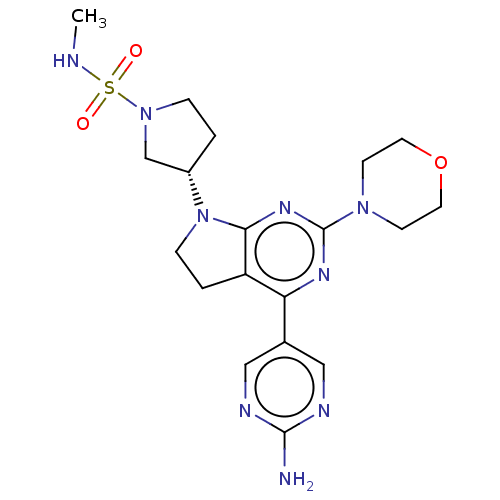
(US9260439, 38)Show SMILES CNS(=O)(=O)N1CC[C@@H](C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C19H27N9O3S/c1-21-32(29,30)27-4-2-14(12-27)28-5-3-15-16(13-10-22-18(20)23-11-13)24-19(25-17(15)28)26-6-8-31-9-7-26/h10-11,14,21H,2-9,12H2,1H3,(H2,20,22,23)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(MOUSE) | BDBM50189257
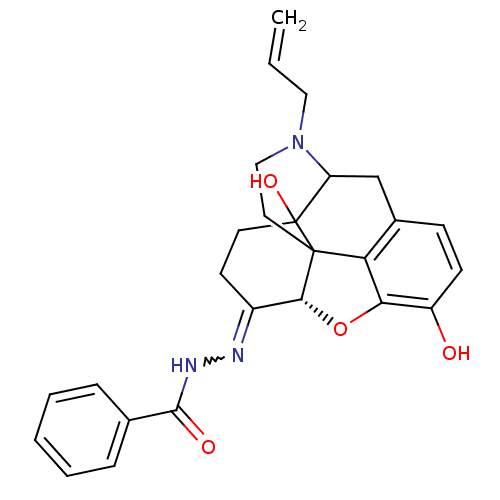
(CHEMBL378753 | NalBzOH)Show SMILES Oc1ccc2CC3N(CC=C)CCC45[C@H](Oc1c24)C(CCC35O)=NNC(=O)c1ccccc1 |w:23.28,TLB:22:21:7.11.12:17.5.4,THB:8:7:21:17.5.4,16:17:21:7.11.12| Show InChI InChI=1S/C26H27N3O4/c1-2-13-29-14-12-25-21-17-8-9-19(30)22(21)33-23(25)18(10-11-26(25,32)20(29)15-17)27-28-24(31)16-6-4-3-5-7-16/h2-9,20,23,30,32H,1,10-15H2,(H,28,31)/t20?,23-,25?,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340347
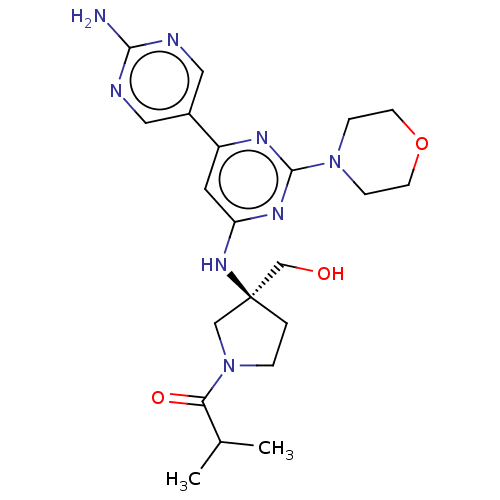
(US9758538, Example 35)Show SMILES CC(C)C(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C21H30N8O3/c1-14(2)18(31)29-4-3-21(12-29,13-30)27-17-9-16(15-10-23-19(22)24-11-15)25-20(26-17)28-5-7-32-8-6-28/h9-11,14,30H,3-8,12-13H2,1-2H3,(H2,22,23,24)(H,25,26,27)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
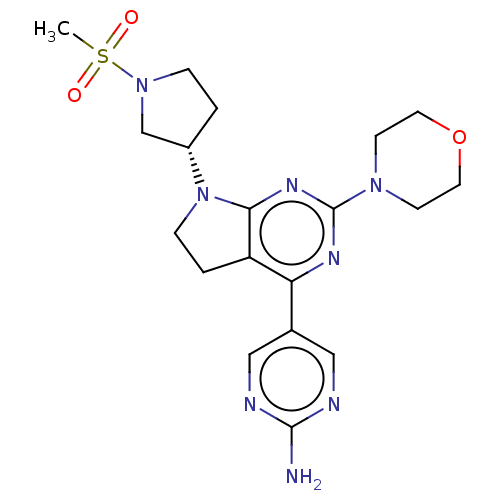
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207043
(US9260439, 20)Show SMILES CS(=O)(=O)N1CC[C@@H](C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C19H26N8O3S/c1-31(28,29)26-4-2-14(12-26)27-5-3-15-16(13-10-21-18(20)22-11-13)23-19(24-17(15)27)25-6-8-30-9-7-25/h10-11,14H,2-9,12H2,1H3,(H2,20,21,22)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409003
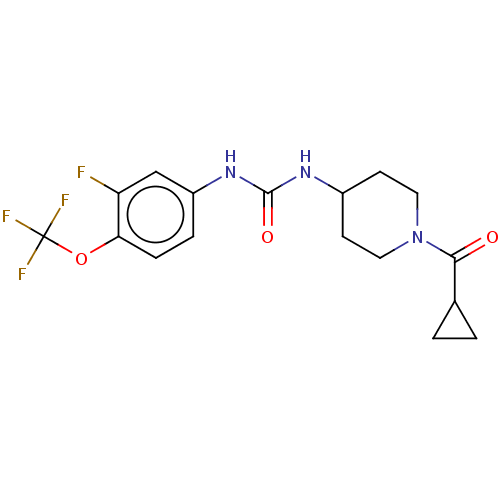
(US10377744, Compound No. 24 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2CC2)ccc1OC(F)(F)F Show InChI InChI=1S/C17H19F4N3O3/c18-13-9-12(3-4-14(13)27-17(19,20)21)23-16(26)22-11-5-7-24(8-6-11)15(25)10-1-2-10/h3-4,9-11H,1-2,5-8H2,(H2,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409003

(US10377744, Compound No. 24 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2CC2)ccc1OC(F)(F)F Show InChI InChI=1S/C17H19F4N3O3/c18-13-9-12(3-4-14(13)27-17(19,20)21)23-16(26)22-11-5-7-24(8-6-11)15(25)10-1-2-10/h3-4,9-11H,1-2,5-8H2,(H2,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115735
BindingDB Entry DOI: 10.7270/Q2SF30T4 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409003

(US10377744, Compound No. 24 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2CC2)ccc1OC(F)(F)F Show InChI InChI=1S/C17H19F4N3O3/c18-13-9-12(3-4-14(13)27-17(19,20)21)23-16(26)22-11-5-7-24(8-6-11)15(25)10-1-2-10/h3-4,9-11H,1-2,5-8H2,(H2,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100521
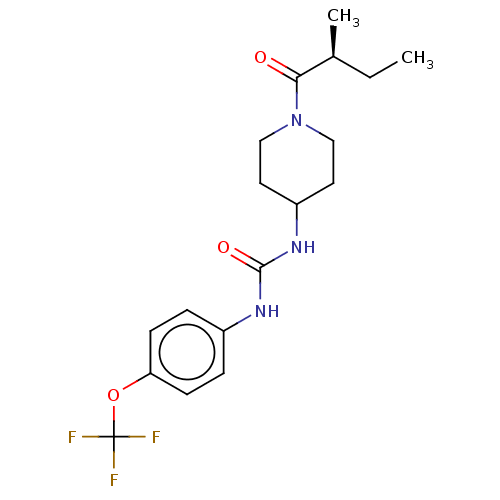
(CHEMBL3327078 | US10377744, Compound No. 2696)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C18H24F3N3O3/c1-3-12(2)16(25)24-10-8-14(9-11-24)23-17(26)22-13-4-6-15(7-5-13)27-18(19,20)21/h4-7,12,14H,3,8-11H2,1-2H3,(H2,22,23,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50189257

(CHEMBL378753 | NalBzOH)Show SMILES Oc1ccc2CC3N(CC=C)CCC45[C@H](Oc1c24)C(CCC35O)=NNC(=O)c1ccccc1 |w:23.28,TLB:22:21:7.11.12:17.5.4,THB:8:7:21:17.5.4,16:17:21:7.11.12| Show InChI InChI=1S/C26H27N3O4/c1-2-13-29-14-12-25-21-17-8-9-19(30)22(21)33-23(25)18(10-11-26(25,32)20(29)15-17)27-28-24(31)16-6-4-3-5-7-16/h2-9,20,23,30,32H,1,10-15H2,(H,28,31)/t20?,23-,25?,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1/2
(Rattus norvegicus (rat)-RAT) | BDBM50219966
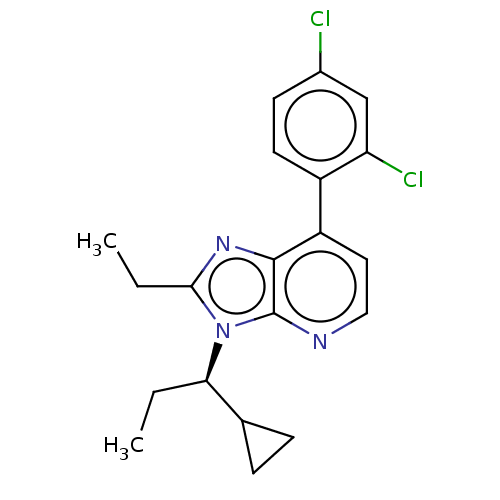
(CHEMBL23959)Show SMILES CC[C@H](C1CC1)n1c(CC)nc2c(ccnc12)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C20H21Cl2N3/c1-3-17(12-5-6-12)25-18(4-2)24-19-15(9-10-23-20(19)25)14-8-7-13(21)11-16(14)22/h7-12,17H,3-6H2,1-2H3/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
In vitro binding affinity to the CRF receptor in rat cortical homogenates |
Bioorg Med Chem Lett 13: 125-8 (2003)
BindingDB Entry DOI: 10.7270/Q2JM2CV9 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Children's Medical Research Center, Tokyo
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 195: 902-9 (1993)
Article DOI: 10.1006/bbrc.1993.2130
BindingDB Entry DOI: 10.7270/Q2FQ9V30 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100519
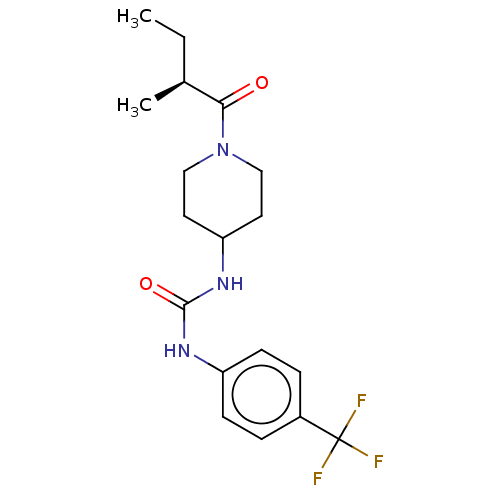
(CHEMBL3327067 | US10377744, Compound No. 2391)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C18H24F3N3O2/c1-3-12(2)16(25)24-10-8-15(9-11-24)23-17(26)22-14-6-4-13(5-7-14)18(19,20)21/h4-7,12,15H,3,8-11H2,1-2H3,(H2,22,23,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50554114
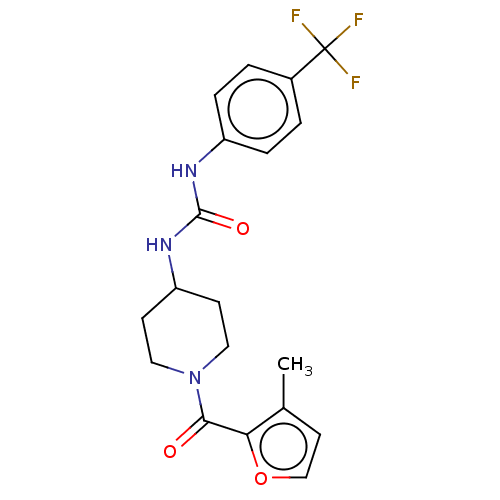
(CHEMBL4781745)Show SMILES Cc1ccoc1C(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115735
BindingDB Entry DOI: 10.7270/Q2SF30T4 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100519

(CHEMBL3327067 | US10377744, Compound No. 2391)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C18H24F3N3O2/c1-3-12(2)16(25)24-10-8-15(9-11-24)23-17(26)22-14-6-4-13(5-7-14)18(19,20)21/h4-7,12,15H,3,8-11H2,1-2H3,(H2,22,23,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human sEH expressed in baculovirus expression system assessed as reduction in ACPU binding incubated for 1 hr by FRET displ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115735
BindingDB Entry DOI: 10.7270/Q2SF30T4 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM408992

(US10377744, Compound No. 13 | US10377744, Compound...)Show SMILES Cc1occc1C(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C19H20F3N3O3/c1-12-16(8-11-28-12)17(26)25-9-6-15(7-10-25)24-18(27)23-14-4-2-13(3-5-14)19(20,21)22/h2-5,8,11,15H,6-7,9-10H2,1H3,(H2,23,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q81H7M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data