| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone-lysine N-methyltransferase, H3 lysine-79 specific |
|---|
| Ligand | BDBM50597863 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2224725 (CHEMBL5138238) |
|---|
| IC50 | 0.300000±n/a nM |
|---|
| Citation |  Talukdar, A; Mukherjee, A; Bhattacharya, D Fascinating Transformation of SAM-Competitive Protein Methyltransferase Inhibitors from Nucleoside Analogues to Non-Nucleoside Analogues. J Med Chem65:1662-1684 (2022) [PubMed] Article Talukdar, A; Mukherjee, A; Bhattacharya, D Fascinating Transformation of SAM-Competitive Protein Methyltransferase Inhibitors from Nucleoside Analogues to Non-Nucleoside Analogues. J Med Chem65:1662-1684 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone-lysine N-methyltransferase, H3 lysine-79 specific |
|---|
| Name: | Histone-lysine N-methyltransferase, H3 lysine-79 specific |
|---|
| Synonyms: | 2.1.1.43 | DOT1-like protein | DOT1-like protein (Dot1L) | DOT1L | DOT1L_HUMAN | H3-K79-HMTase | Histone H3-K79 methyltransferase | Histone H3-K79 methyltransferase (DOT1L) | Histone Methyltransferase DOT1L | Histone-lysine N-methyltransferase, H3 lysine-79 specific (DOT1L) | KIAA1814 | KMT4 | Lysine N-methyltransferase 4 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 184911.91 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q8TEK3 |
|---|
| Residue: | 1537 |
|---|
| Sequence: | MGEKLELRLKSPVGAEPAVYPWPLPVYDKHHDAAHEIIETIRWVCEEIPDLKLAMENYVL
IDYDTKSFESMQRLCDKYNRAIDSIHQLWKGTTQPMKLNTRPSTGLLRHILQQVYNHSVT
DPEKLNNYEPFSPEVYGETSFDLVAQMIDEIKMTDDDLFVDLGSGVGQVVLQVAAATNCK
HHYGVEKADIPAKYAETMDREFRKWMKWYGKKHAEYTLERGDFLSEEWRERIANTSVIFV
NNFAFGPEVDHQLKERFANMKEGGRIVSSKPFAPLNFRINSRNLSDIGTIMRVVELSPLK
GSVSWTGKPVSYYLHTIDRTILENYFSSLKNPKLREEQEAARRRQQRESKSNAATPTKGP
EGKVAGPADAPMDSGAEEEKAGAATVKKPSPSKARKKKLNKKGRKMAGRKRGRPKKMNTA
NPERKPKKNQTALDALHAQTVSQTAASSPQDAYRSPHSPFYQLPPSVQRHSPNPLLVAPT
PPALQKLLESFKIQYLQFLAYTKTPQYKASLQELLGQEKEKNAQLLGAAQQLLSHCQAQK
EEIRRLFQQKLDELGVKALTYNDLIQAQKEISAHNQQLREQSEQLEQDNRALRGQSLQLL
KARCEELQLDWATLSLEKLLKEKQALKSQISEKQRHCLELQISIVELEKSQRQQELLQLK
SCVPPDDALSLHLRGKGALGRELEPDASRLHLELDCTKFSLPHLSSMSPELSMNGQAAGY
ELCGVLSRPSSKQNTPQYLASPLDQEVVPCTPSHVGRPRLEKLSGLAAPDYTRLSPAKIV
LRRHLSQDHTVPGRPAASELHSRAEHTKENGLPYQSPSVPGSMKLSPQDPRPLSPGALQL
AGEKSSEKGLRERAYGSSGELITSLPISIPLSTVQPNKLPVSIPLASVVLPSRAERARST
PSPVLQPRDPSSTLEKQIGANAHGAGSRSLALAPAGFSYAGSVAISGALAGSPASLTPGA
EPATLDESSSSGSLFATVGSRSSTPQHPLLLAQPRNSLPASPAHQLSSSPRLGGAAQGPL
PEASKGDLPSDSGFSDPESEAKRRIVFTITTGAGSAKQSPSSKHSPLTASARGDCVPSHG
QDSRRRGRRKRASAGTPSLSAGVSPKRRALPSVAGLFTQPSGSPLNLNSMVSNINQPLEI
TAISSPETSLKSSPVPYQDHDQPPVLKKERPLSQTNGAHYSPLTSDEEPGSEDEPSSARI
ERKIATISLESKSPPKTLENGGGLAGRKPAPAGEPVNSSKWKSTFSPISDIGLAKSADSP
LQASSALSQNSLFTFRPALEEPSADAKLAAHPRKGFPGSLSGADGLSPGTNPANGCTFGG
GLAADLSLHSFSDGASLPHKGPEAAGLSSPLSFPSQRGKEGSDANPFLSKRQLDGLAGLK
GEGSRGKEAGEGGLPLCGPTDKTPLLSGKAAKARDREVDLKNGHNLFISAAAVPPGSLLS
GPGLAPAASSAGGAASSAQTHRSFLGPFPPGPQFALGPMSLQANLGSVAGSSVLQSLFSS
VPAAAGLVHVSSAATRLTNSHAMGSFSGVAGGTVGGN
|
|
|
|---|
| BDBM50597863 |
|---|
| n/a |
|---|
| Name | BDBM50597863 |
|---|
| Synonyms: | CHEMBL5188291 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H40BrN7O4 |
|---|
| Mol. Mass. | 618.566 |
|---|
| SMILES | CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cc(Br)c2c(N)ncnc12 |r| |
|---|
| Structure | 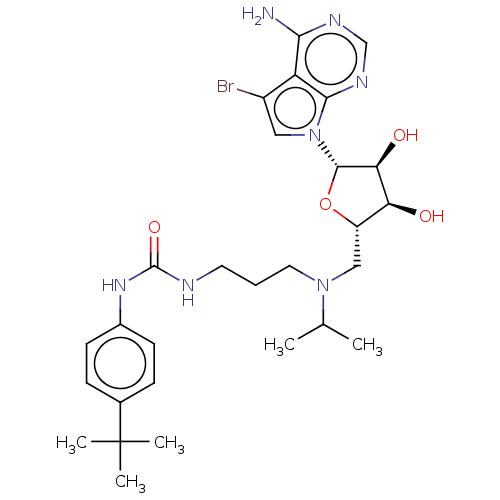
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Talukdar, A; Mukherjee, A; Bhattacharya, D Fascinating Transformation of SAM-Competitive Protein Methyltransferase Inhibitors from Nucleoside Analogues to Non-Nucleoside Analogues. J Med Chem65:1662-1684 (2022) [PubMed] Article
Talukdar, A; Mukherjee, A; Bhattacharya, D Fascinating Transformation of SAM-Competitive Protein Methyltransferase Inhibitors from Nucleoside Analogues to Non-Nucleoside Analogues. J Med Chem65:1662-1684 (2022) [PubMed] Article