 Found 792 hits with Last Name = 'talukdar' and Initial = 'a'
Found 792 hits with Last Name = 'talukdar' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50597886

(CHEMBL5180177)Show SMILES [H][C@]1(O[C@@H]([C@@H](O)[C@H]1O)n1ccc2c(N)ncnc12)[C@H](O)c1ccc(Cl)c(F)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597868

(CHEMBL5202438)Show SMILES CC(C)N(C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12)C1CC(CCc2nc3cc(ccc3[nH]2)C(C)(C)C)C1 |r,wU:5.4,7.12,wD:8.8,10.11,(-4.59,1.12,;-3.05,1.12,;-2.28,2.45,;-2.28,-.21,;-3.05,-1.55,;-4.59,-1.55,;-5.62,-.4,;-7.02,-1.03,;-6.86,-2.56,;-7.95,-3.65,;-5.35,-2.88,;-4.59,-4.21,;-8.29,-.15,;-8.4,1.38,;-9.89,1.75,;-10.71,.44,;-12.23,.17,;-13.22,1.35,;-12.75,-1.29,;-11.75,-2.46,;-10.24,-2.19,;-9.71,-.73,;-.74,-.21,;.37,.89,;1.46,-.2,;3,-.2,;3.77,1.14,;5.31,1.14,;6.22,2.38,;7.68,1.91,;9.01,2.67,;10.34,1.9,;10.35,.37,;9.02,-.4,;7.68,.37,;6.22,-.11,;11.68,2.67,;11.68,4.21,;12.45,1.34,;13.22,2.67,;.35,-1.3,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50597877
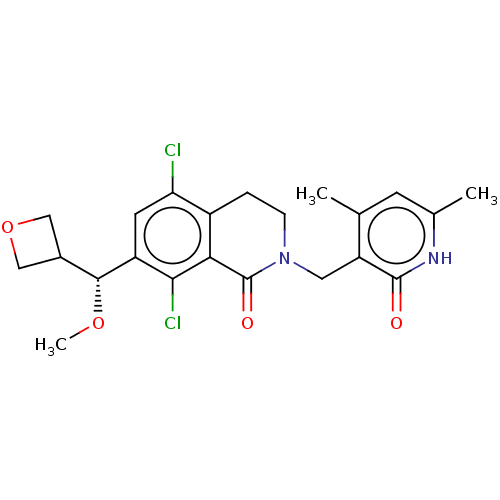
(CHEMBL5191399)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193709
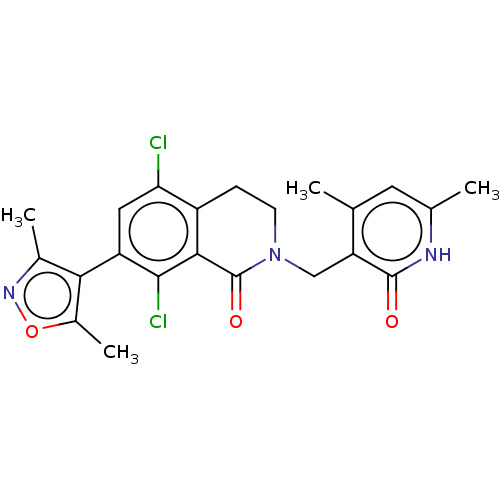
(CHEMBL3911017)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(59.06,-26.01,;58.74,-27.52,;59.77,-28.66,;59,-30,;57.5,-29.68,;56.35,-30.71,;57.33,-28.15,;55.99,-27.38,;54.66,-28.14,;53.34,-27.37,;52.01,-28.14,;53.35,-25.85,;52.02,-25.09,;52.02,-23.55,;53.35,-22.77,;53.35,-21.23,;52.02,-20.46,;52.02,-18.92,;53.36,-18.16,;50.7,-18.15,;49.36,-18.92,;48.03,-18.14,;49.35,-20.46,;50.69,-21.24,;50.69,-22.78,;54.68,-23.55,;56.01,-22.79,;54.68,-25.09,;55.99,-25.86,;57.33,-25.09,)| Show InChI InChI=1S/C22H21Cl2N3O3/c1-10-7-11(2)25-21(28)16(10)9-27-6-5-14-17(23)8-15(20(24)19(14)22(27)29)18-12(3)26-30-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597860

(CHEMBL5172325)Show SMILES N[C@@H](CCSC[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50017292
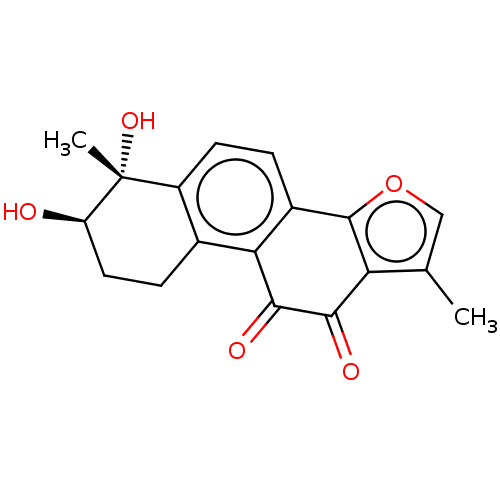
(CHEMBL3287734)Show SMILES Cc1coc-2c1C(=O)C(=O)c1c-2ccc2c1CC[C@@H](O)[C@]2(C)O |r| Show InChI InChI=1S/C18H16O5/c1-8-7-23-17-10-3-5-11-9(4-6-12(19)18(11,2)22)14(10)16(21)15(20)13(8)17/h3,5,7,12,19,22H,4,6H2,1-2H3/t12-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597869

(CHEMBL5194696)Show SMILES CNc1ncnc2n(cnc12)[C@H]1O[C@@H](CSCC[C@H](N)C(O)=O)[C@H](O)[C@@H]1O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316579
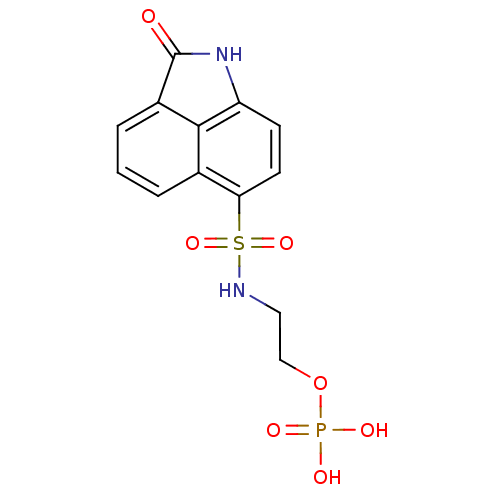
(2-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C13H13N2O7PS/c16-13-9-3-1-2-8-11(5-4-10(15-13)12(8)9)24(20,21)14-6-7-22-23(17,18)19/h1-5,14H,6-7H2,(H,15,16)(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316577
(4-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C15H17N2O7PS/c18-15-11-5-3-4-10-13(7-6-12(17-15)14(10)11)26(22,23)16-8-1-2-9-24-25(19,20)21/h3-7,16H,1-2,8-9H2,(H,17,18)(H2,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
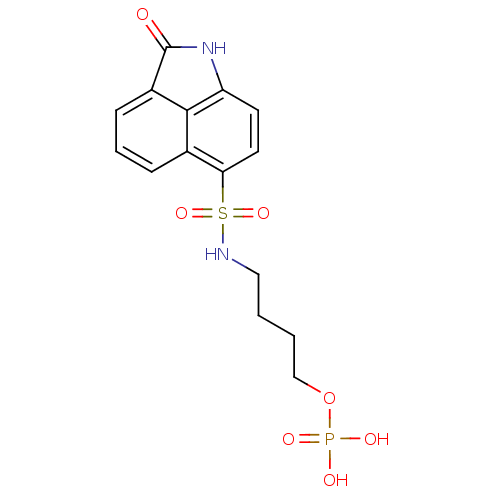
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316578
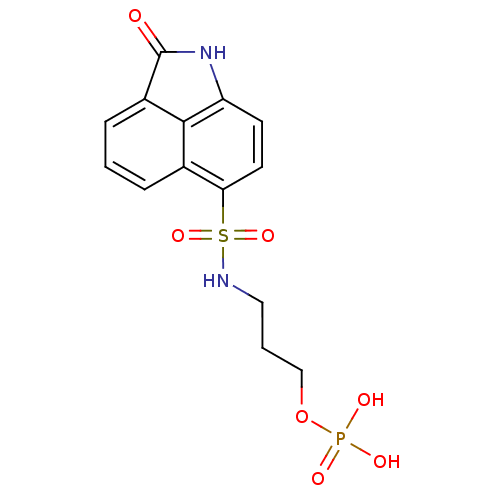
(3-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C14H15N2O7PS/c17-14-10-4-1-3-9-12(6-5-11(16-14)13(9)10)25(21,22)15-7-2-8-23-24(18,19)20/h1,3-6,15H,2,7-8H2,(H,16,17)(H2,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316576
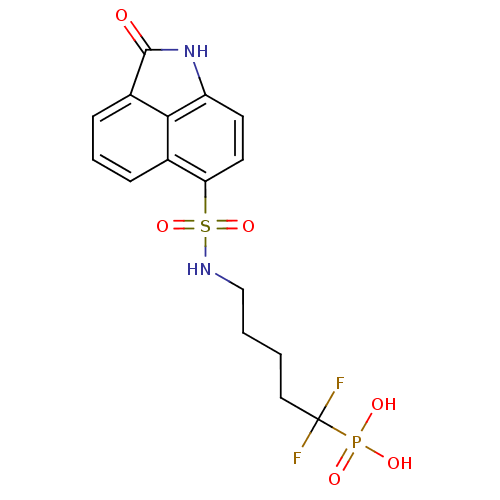
(1,1-difluoro-5-(2-oxo-1,2-dihydrobenzo[cd]indole-6...)Show SMILES OP(O)(=O)C(F)(F)CCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C16H17F2N2O6PS/c17-16(18,27(22,23)24)8-1-2-9-19-28(25,26)13-7-6-12-14-10(13)4-3-5-11(14)15(21)20-12/h3-7,19H,1-2,8-9H2,(H,20,21)(H2,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316580

(6-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCCCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C17H21N2O7PS/c20-17-13-7-5-6-12-15(9-8-14(19-17)16(12)13)28(24,25)18-10-3-1-2-4-11-26-27(21,22)23/h5-9,18H,1-4,10-11H2,(H,19,20)(H2,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316581
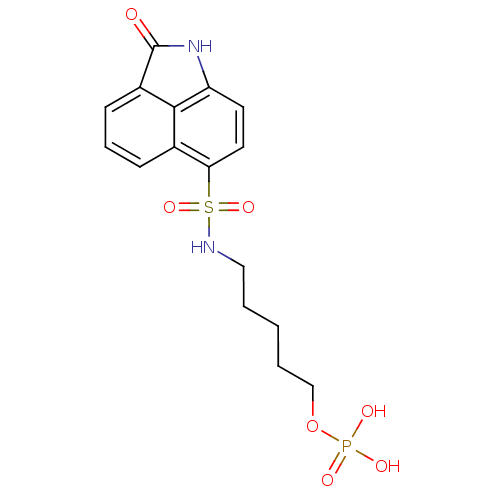
(5-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C16H19N2O7PS/c19-16-12-6-4-5-11-14(8-7-13(18-16)15(11)12)27(23,24)17-9-2-1-3-10-25-26(20,21)22/h4-8,17H,1-3,9-10H2,(H,18,19)(H2,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316575
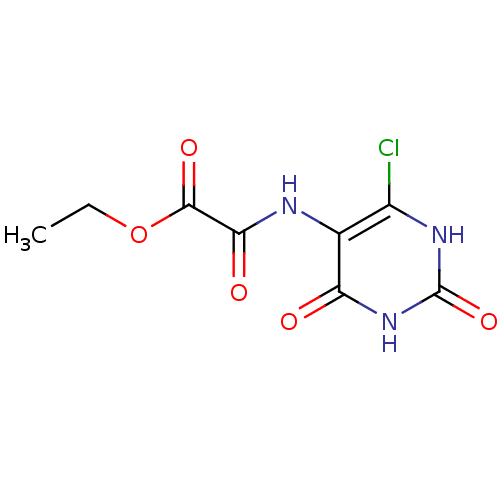
(CHEMBL1095229 | Ethyl2-(6-chloro-2,4,dioxo-1,2,3,4...)Show InChI InChI=1S/C8H8ClN3O5/c1-2-17-7(15)6(14)10-3-4(9)11-8(16)12-5(3)13/h2H2,1H3,(H,10,14)(H2,11,12,13,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316577

(4-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C15H17N2O7PS/c18-15-11-5-3-4-10-13(7-6-12(17-15)14(10)11)26(22,23)16-8-1-2-9-24-25(19,20)21/h3-7,16H,1-2,8-9H2,(H,17,18)(H2,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-inhibitor complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316574

(2-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show InChI InChI=1S/C13H10N2O5S/c16-11(17)6-14-21(19,20)10-5-4-9-12-7(10)2-1-3-8(12)13(18)15-9/h1-5,14H,6H2,(H,15,18)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316574

(2-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show InChI InChI=1S/C13H10N2O5S/c16-11(17)6-14-21(19,20)10-5-4-9-12-7(10)2-1-3-8(12)13(18)15-9/h1-5,14H,6H2,(H,15,18)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-inhibitor complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316578

(3-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C14H15N2O7PS/c17-14-10-4-1-3-9-12(6-5-11(16-14)13(9)10)25(21,22)15-7-2-8-23-24(18,19)20/h1,3-6,15H,2,7-8H2,(H,16,17)(H2,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-inhibitor complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316579

(2-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C13H13N2O7PS/c16-13-9-3-1-2-8-11(5-4-10(15-13)12(8)9)24(20,21)14-6-7-22-23(17,18)19/h1-5,14H,6-7H2,(H,15,16)(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316579

(2-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C13H13N2O7PS/c16-13-9-3-1-2-8-11(5-4-10(15-13)12(8)9)24(20,21)14-6-7-22-23(17,18)19/h1-5,14H,6-7H2,(H,15,16)(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-inhibitor complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316578

(3-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C14H15N2O7PS/c17-14-10-4-1-3-9-12(6-5-11(16-14)13(9)10)25(21,22)15-7-2-8-23-24(18,19)20/h1,3-6,15H,2,7-8H2,(H,16,17)(H2,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316576

(1,1-difluoro-5-(2-oxo-1,2-dihydrobenzo[cd]indole-6...)Show SMILES OP(O)(=O)C(F)(F)CCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C16H17F2N2O6PS/c17-16(18,27(22,23)24)8-1-2-9-19-28(25,26)13-7-6-12-14-10(13)4-3-5-11(14)15(21)20-12/h3-7,19H,1-2,8-9H2,(H,20,21)(H2,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316581

(5-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C16H19N2O7PS/c19-16-12-6-4-5-11-14(8-7-13(18-16)15(11)12)27(23,24)17-9-2-1-3-10-25-26(20,21)22/h4-8,17H,1-3,9-10H2,(H,18,19)(H2,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316577

(4-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C15H17N2O7PS/c18-15-11-5-3-4-10-13(7-6-12(17-15)14(10)11)26(22,23)16-8-1-2-9-24-25(19,20)21/h3-7,16H,1-2,8-9H2,(H,17,18)(H2,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316580

(6-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...)Show SMILES OP(O)(=O)OCCCCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C17H21N2O7PS/c20-17-13-7-5-6-12-15(9-8-14(19-17)16(12)13)28(24,25)18-10-3-1-2-4-11-26-27(21,22)23/h5-9,18H,1-4,10-11H2,(H,19,20)(H2,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316575

(CHEMBL1095229 | Ethyl2-(6-chloro-2,4,dioxo-1,2,3,4...)Show InChI InChI=1S/C8H8ClN3O5/c1-2-17-7(15)6(14)10-3-4(9)11-8(16)12-5(3)13/h2H2,1H3,(H,10,14)(H2,11,12,13,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-inhibitor complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316576

(1,1-difluoro-5-(2-oxo-1,2-dihydrobenzo[cd]indole-6...)Show SMILES OP(O)(=O)C(F)(F)CCCCNS(=O)(=O)c1ccc2NC(=O)c3cccc1c23 Show InChI InChI=1S/C16H17F2N2O6PS/c17-16(18,27(22,23)24)8-1-2-9-19-28(25,26)13-7-6-12-14-10(13)4-3-5-11(14)15(21)20-12/h3-7,19H,1-2,8-9H2,(H,20,21)(H2,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-inhibitor complex state after 30 mins by fluorescence assay |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
6,7-dimethyl-8-ribityllumazine synthase
(Mycobacterium tuberculosis) | BDBM50316575

(CHEMBL1095229 | Ethyl2-(6-chloro-2,4,dioxo-1,2,3,4...)Show InChI InChI=1S/C8H8ClN3O5/c1-2-17-7(15)6(14)10-3-4(9)11-8(16)12-5(3)13/h2H2,1H3,(H,10,14)(H2,11,12,13,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... |
Bioorg Med Chem 18: 3518-34 (2010)
Article DOI: 10.1016/j.bmc.2010.03.072
BindingDB Entry DOI: 10.7270/Q29P31S5 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823
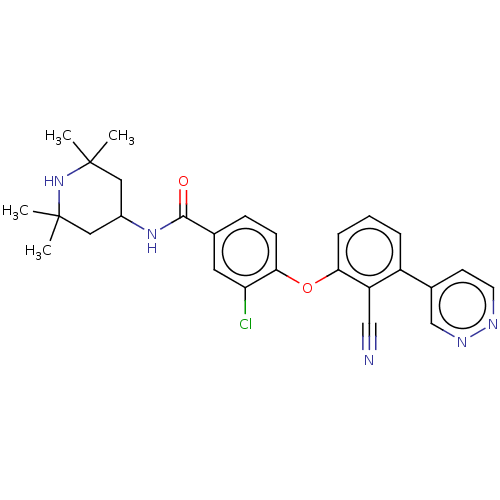
(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50597874
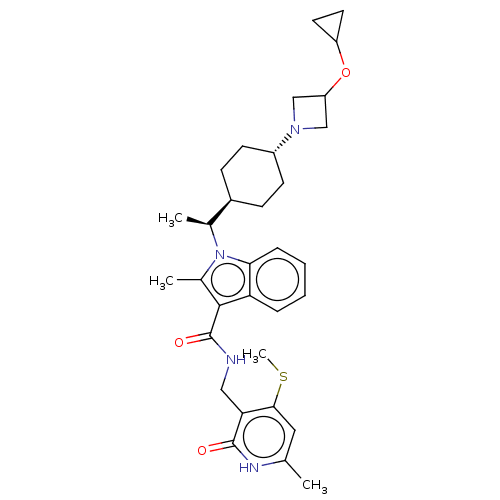
(CHEMBL5188476)Show SMILES [H][C@@]1(CC[C@@H](CC1)N1CC(C1)OC1CC1)[C@H](C)n1c(C)c(C(=O)NCc2c(SC)cc(C)[nH]c2=O)c2ccccc12 |r,wU:15.18,wD:4.7,1.0,(.87,-.92,;-.67,-.92,;.1,-2.26,;-.67,-3.59,;-2.21,-3.59,;-2.98,-2.26,;-2.21,-.92,;-2.98,-4.92,;-2.58,-6.44,;-4.07,-6.83,;-4.47,-5.32,;-4.84,-8.17,;-4.07,-9.5,;-4.07,-11.05,;-2.73,-10.27,;.1,.41,;1.64,.41,;-.67,1.75,;-.2,3.21,;1.29,3.61,;-1.44,4.11,;-1.44,5.65,;-2.78,6.43,;-.11,6.43,;-.11,7.97,;1.22,8.74,;2.56,7.97,;2.56,6.43,;3.89,5.66,;3.89,8.74,;3.89,10.28,;5.22,11.05,;2.56,11.05,;1.22,10.28,;-.11,11.05,;-2.69,3.21,;-4.2,3.53,;-5.22,2.38,;-4.75,.93,;-3.24,.61,;-2.21,1.75,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50597880
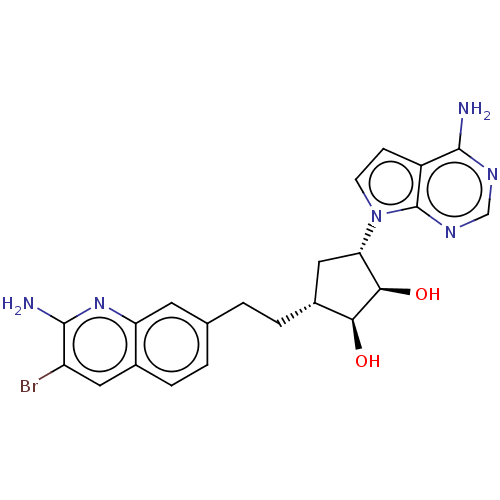
(CHEMBL5203719)Show SMILES Nc1nc2cc(CC[C@@H]3C[C@@H]([C@@H](O)[C@H]3O)n3ccc4c(N)ncnc34)ccc2cc1Br |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597863
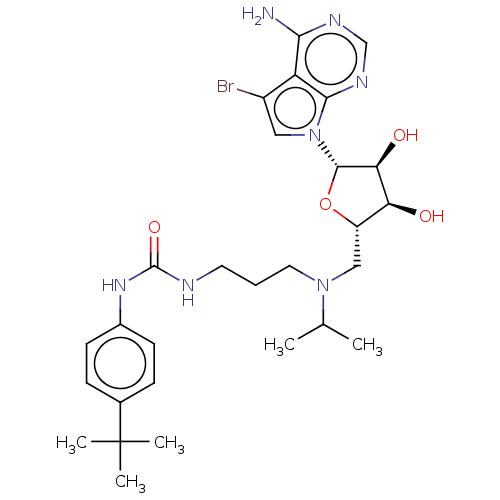
(CHEMBL5188291)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cc(Br)c2c(N)ncnc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50185219
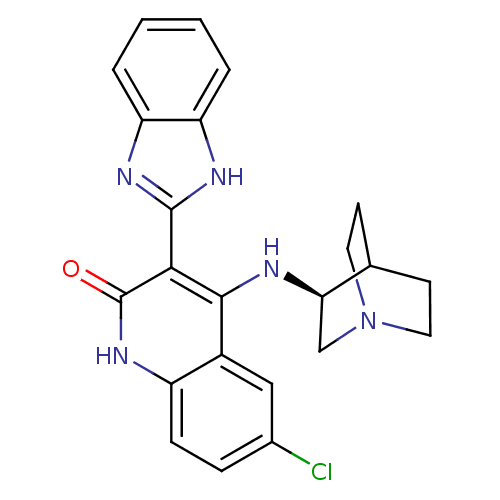
((S)-3-(1H-benzo[d]imidazol-2-yl)-6-chloro-4-(quinu...)Show SMILES Clc1ccc2[nH]c(=O)c(-c3nc4ccccc4[nH]3)c(N[C@@H]3CN4CCC3CC4)c2c1 |wU:20.21,TLB:19:20:24.23:26.27,(4.27,-32.71,;5.6,-33.48,;5.6,-35.02,;6.93,-35.79,;8.27,-35.03,;9.61,-35.79,;10.95,-35.01,;12.28,-35.78,;10.94,-33.46,;12.27,-32.69,;13.68,-33.32,;14.7,-32.18,;16.24,-32.18,;17.02,-30.84,;16.23,-29.5,;14.69,-29.51,;13.93,-30.84,;12.43,-31.16,;9.59,-32.69,;9.59,-31.15,;8.64,-29.94,;8.36,-28.54,;7.01,-27.94,;5.55,-28.58,;5.74,-29.96,;7.27,-29.3,;7.53,-27.41,;7.08,-26.31,;8.26,-33.47,;6.93,-32.71,)| Show InChI InChI=1S/C23H22ClN5O/c24-14-5-6-16-15(11-14)21(25-19-12-29-9-7-13(19)8-10-29)20(23(30)28-16)22-26-17-3-1-2-4-18(17)27-22/h1-6,11,13,19H,7-10,12H2,(H,26,27)(H2,25,28,30)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114304
BindingDB Entry DOI: 10.7270/Q25B06GC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597867
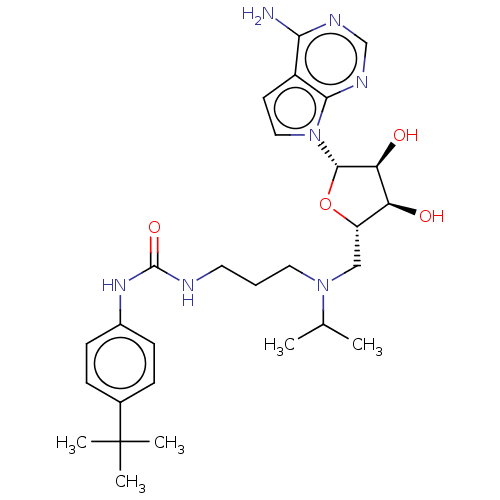
(CHEMBL5176983)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1ccc2c(N)ncnc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597863

(CHEMBL5188291)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cc(Br)c2c(N)ncnc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50238541
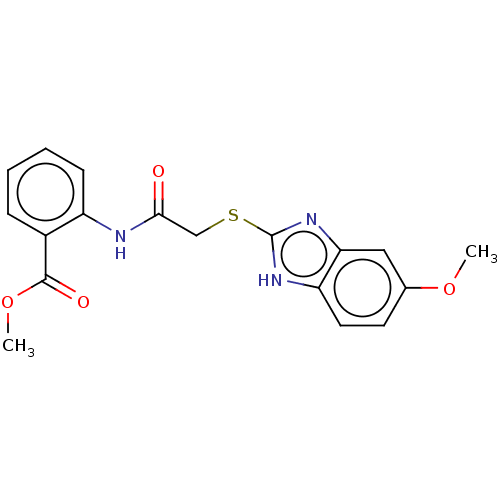
(CHEMBL4092336)Show SMILES COC(=O)c1ccccc1NC(=O)CSc1nc2cc(OC)ccc2[nH]1 Show InChI InChI=1S/C18H17N3O4S/c1-24-11-7-8-14-15(9-11)21-18(20-14)26-10-16(22)19-13-6-4-3-5-12(13)17(23)25-2/h3-9H,10H2,1-2H3,(H,19,22)(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50597881
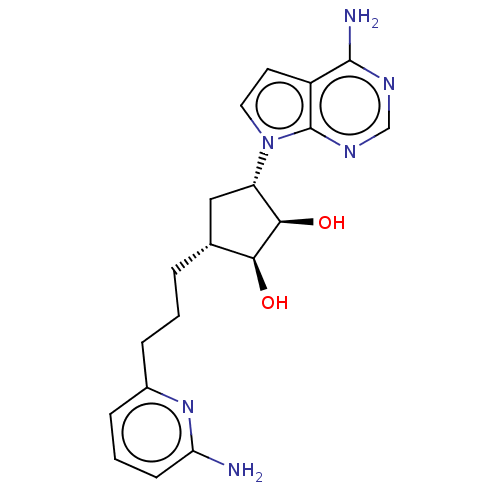
(CHEMBL5187591)Show SMILES Nc1cccc(CCC[C@@H]2C[C@@H]([C@@H](O)[C@H]2O)n2ccc3c(N)ncnc23)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536826

(CHEMBL4590355)Show SMILES CNc1ccnc(Nc2ccc3cc(C)n(-c4ccccc4Oc4cnc5n(C)cnc5c4)c3c2)n1 |(29.26,-10.25,;27.93,-11.02,;27.94,-12.56,;29.28,-13.33,;29.28,-14.87,;27.95,-15.64,;26.62,-14.88,;25.29,-15.64,;23.96,-14.88,;23.95,-13.33,;22.62,-12.56,;21.29,-13.34,;19.82,-12.85,;18.9,-14.1,;17.36,-14.08,;19.8,-15.36,;19.31,-16.81,;20.33,-17.95,;19.85,-19.4,;18.34,-19.72,;17.32,-18.56,;17.81,-17.11,;16.79,-15.96,;15.29,-16.26,;14.81,-17.71,;13.31,-18.01,;12.29,-16.86,;10.74,-16.84,;9.83,-18.07,;10.28,-15.37,;11.54,-14.47,;12.78,-15.39,;14.28,-15.1,;21.28,-14.89,;22.62,-15.65,;26.61,-13.34,)| Show InChI InChI=1S/C27H24N8O/c1-17-12-18-8-9-19(32-27-29-11-10-25(28-2)33-27)13-23(18)35(17)22-6-4-5-7-24(22)36-20-14-21-26(30-15-20)34(3)16-31-21/h4-16H,1-3H3,(H2,28,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Toll-like receptor 8
(Homo sapiens (Human)) | BDBM50552139
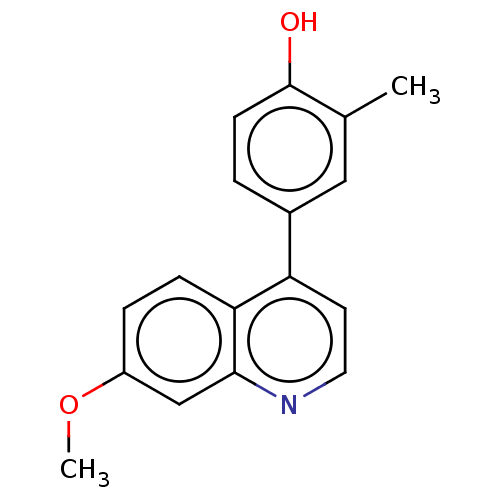
(CHEMBL4790706) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor in rat aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Toll-like receptor 8
(Homo sapiens (Human)) | BDBM50587871
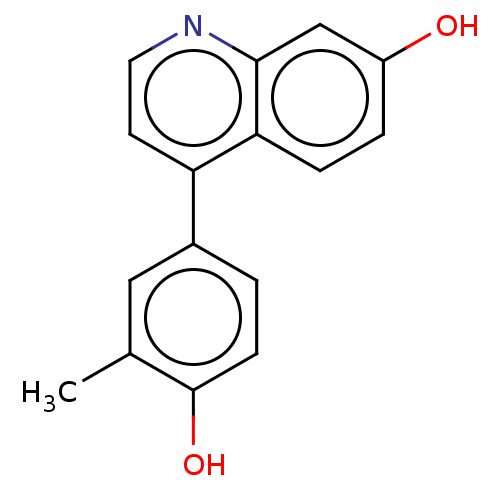
(CHEMBL5176610) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor in rat aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400778
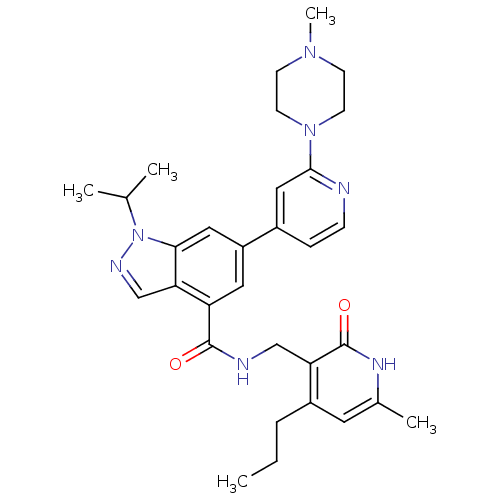
(CHEMBL2204995)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccnc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-23-14-21(4)35-31(40)26(23)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)22-8-9-32-29(17-22)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H2
(Homo sapiens (Human)) | BDBM423017
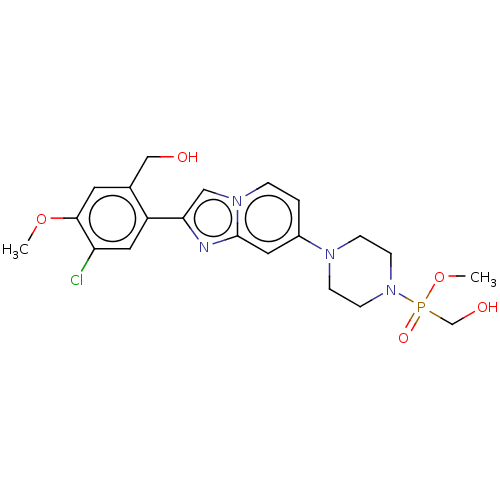
(US10508109, Example 203 | dimethyl (4-(2-(5-chloro...)Show SMILES COc1cc(CO)c(cc1Cl)-c1cn2ccc(cc2n1)N1CCN(CC1)P(=O)(CO)OC Show InChI InChI=1S/C21H26ClN4O5P/c1-30-20-9-15(13-27)17(11-18(20)22)19-12-25-4-3-16(10-21(25)23-19)24-5-7-26(8-6-24)32(29,14-28)31-2/h3-4,9-12,27-28H,5-8,13-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.77 | n/a | n/a | n/a | n/a | n/a | n/a |
ONCOTHERAPY SCIENCE, INC.
US Patent
| Assay Description
Assay buffer (4 μL) was added into all the wells, including test compound and control wells (except for Sinefungin control wells), using the Mul... |
US Patent US10508109 (2019)
BindingDB Entry DOI: 10.7270/Q2H997K9 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50110357
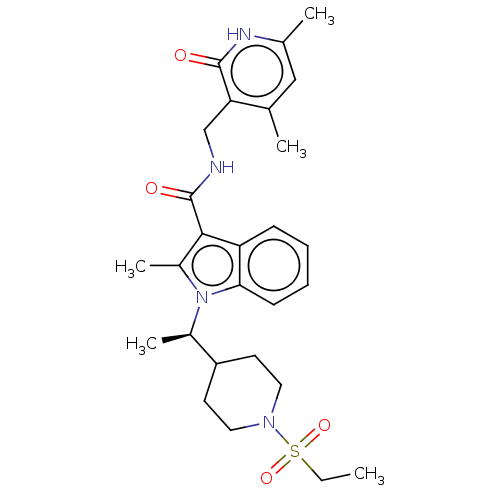
(CHEMBL3605455)Show SMILES CCS(=O)(=O)N1CCC(CC1)[C@@H](C)n1c(C)c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H2
(Homo sapiens (Human)) | BDBM423023

(3-(2-(7-(1,4-diazepan-1- yl)imidazo[1,2-a]pyridine...)Show SMILES COc1cc(OCCCN(C)C)c(cc1Cl)-c1cn2ccc(cc2n1)N1CCCNCC1 Show InChI InChI=1S/C24H32ClN5O2/c1-28(2)9-5-13-32-22-16-23(31-3)20(25)15-19(22)21-17-30-11-6-18(14-24(30)27-21)29-10-4-7-26-8-12-29/h6,11,14-17,26H,4-5,7-10,12-13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.07 | n/a | n/a | n/a | n/a | n/a | n/a |
ONCOTHERAPY SCIENCE, INC.
US Patent
| Assay Description
Assay buffer (4 μL) was added into all the wells, including test compound and control wells (except for Sinefungin control wells), using the Mul... |
US Patent US10508109 (2019)
BindingDB Entry DOI: 10.7270/Q2H997K9 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H2
(Homo sapiens (Human)) | BDBM423039
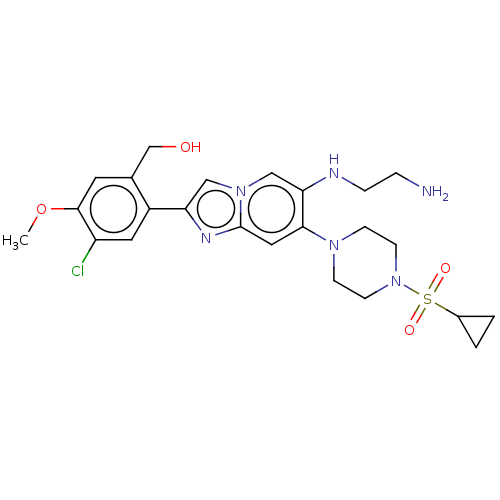
(N1-(2-(5-chloro-2,4- dimethoxyphenyl)-7-(4- (cyclo...)Show SMILES COc1cc(CO)c(cc1Cl)-c1cn2cc(NCCN)c(cc2n1)N1CCN(CC1)S(=O)(=O)C1CC1 Show InChI InChI=1S/C24H31ClN6O4S/c1-35-23-10-16(15-32)18(11-19(23)25)20-13-30-14-21(27-5-4-26)22(12-24(30)28-20)29-6-8-31(9-7-29)36(33,34)17-2-3-17/h10-14,17,27,32H,2-9,15,26H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ONCOTHERAPY SCIENCE, INC.
US Patent
| Assay Description
Assay buffer (4 μL) was added into all the wells, including test compound and control wells (except for Sinefungin control wells), using the Mul... |
US Patent US10508109 (2019)
BindingDB Entry DOI: 10.7270/Q2H997K9 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 7
(Homo sapiens (Human)) | BDBM50597884

(CHEMBL5185202)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCCNCc2cccc(c2)-c2ccc(Cl)cc2)[C@@H](O)[C@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase SUV39H2
(Homo sapiens (Human)) | BDBM422884

(N1-(2-(5-chloro-2,4- dimethoxyphenyl)-7- methoxyim...)Show InChI InChI=1S/C18H21ClN4O3/c1-26-17-4-11(9-24)13(6-14(17)19)16-8-23-7-15(21-3-2-20)12(10-25)5-18(23)22-16/h4-8,21,24-25H,2-3,9-10,20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.71 | n/a | n/a | n/a | n/a | n/a | n/a |
ONCOTHERAPY SCIENCE, INC.
US Patent
| Assay Description
Assay buffer (4 μL) was added into all the wells, including test compound and control wells (except for Sinefungin control wells), using the Mul... |
US Patent US10508109 (2019)
BindingDB Entry DOI: 10.7270/Q2H997K9 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H2
(Homo sapiens (Human)) | BDBM423024
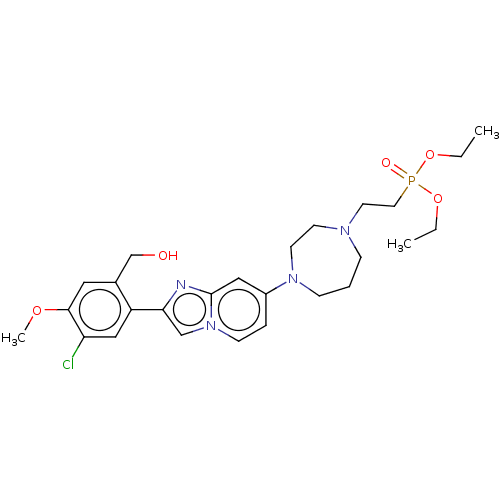
(US10508109, Example 210 | diethyl (2-(4-(2-(5-chlo...)Show SMILES CCOP(=O)(CCN1CCCN(CC1)c1ccn2cc(nc2c1)-c1cc(Cl)c(OC)cc1CO)OCC Show InChI InChI=1S/C26H36ClN4O5P/c1-4-35-37(33,36-5-2)14-13-29-8-6-9-30(12-11-29)21-7-10-31-18-24(28-26(31)16-21)22-17-23(27)25(34-3)15-20(22)19-32/h7,10,15-18,32H,4-6,8-9,11-14,19H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ONCOTHERAPY SCIENCE, INC.
US Patent
| Assay Description
Assay buffer (4 μL) was added into all the wells, including test compound and control wells (except for Sinefungin control wells), using the Mul... |
US Patent US10508109 (2019)
BindingDB Entry DOI: 10.7270/Q2H997K9 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H2
(Homo sapiens (Human)) | BDBM422944

(3-(4-(2-(5-bromo-2,4- dimethoxyphenyl)imidazo[1,2-...)Show SMILES COc1cc(CO)c(cc1Br)-c1cn2ccc(cc2n1)N1CCN(CCCN(C)C)CC1 Show InChI InChI=1S/C24H32BrN5O2/c1-27(2)6-4-7-28-9-11-29(12-10-28)19-5-8-30-16-22(26-24(30)14-19)20-15-21(25)23(32-3)13-18(20)17-31/h5,8,13-16,31H,4,6-7,9-12,17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ONCOTHERAPY SCIENCE, INC.
US Patent
| Assay Description
Assay buffer (4 μL) was added into all the wells, including test compound and control wells (except for Sinefungin control wells), using the Mul... |
US Patent US10508109 (2019)
BindingDB Entry DOI: 10.7270/Q2H997K9 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H2
(Homo sapiens (Human)) | BDBM422805
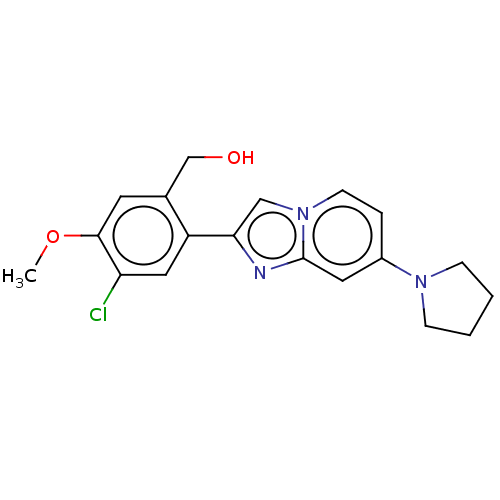
(2-(5-chloro-2,4- dimethoxyphenyl)-7-(pyrrolidin- 1...)Show InChI InChI=1S/C19H20ClN3O2/c1-25-18-8-13(12-24)15(10-16(18)20)17-11-23-7-4-14(9-19(23)21-17)22-5-2-3-6-22/h4,7-11,24H,2-3,5-6,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONCOTHERAPY SCIENCE, INC.
US Patent
| Assay Description
Assay buffer (4 μL) was added into all the wells, including test compound and control wells (except for Sinefungin control wells), using the Mul... |
US Patent US10508109 (2019)
BindingDB Entry DOI: 10.7270/Q2H997K9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data