| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein kinase Lck |
|---|
| Ligand | BDBM50186560 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_376957 (CHEMBL864124) |
|---|
| IC50 | 3±n/a nM |
|---|
| Citation |  Maier, JA; Brugel, TA; Sabat, M; Golebiowski, A; Laufersweiler, MJ; VanRens, JC; Hopkins, CR; De, B; Hsieh, LC; Brown, KK; Easwaran, V; Janusz, MJ Development of N-4,6-pyrimidine-N-alkyl-N'-phenyl ureas as orally active inhibitors of lymphocyte specific tyrosine kinase. Bioorg Med Chem Lett16:3646-50 (2006) [PubMed] Article Maier, JA; Brugel, TA; Sabat, M; Golebiowski, A; Laufersweiler, MJ; VanRens, JC; Hopkins, CR; De, B; Hsieh, LC; Brown, KK; Easwaran, V; Janusz, MJ Development of N-4,6-pyrimidine-N-alkyl-N'-phenyl ureas as orally active inhibitors of lymphocyte specific tyrosine kinase. Bioorg Med Chem Lett16:3646-50 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein kinase Lck |
|---|
| Name: | Tyrosine-protein kinase Lck |
|---|
| Synonyms: | 2.7.10.2 | LCK | LCK_HUMAN | LSK | Leukocyte C-terminal Src kinase | Lymphocyte cell-specific protein-tyrosine kinase | Lymphocyte-specific protein tyrosine kinase | P56-LCK | Protein YT16 | Proto-oncogene Lck | Proto-oncogene tyrosine-protein kinase LCK | Src/Lck kinase | T cell-specific protein-tyrosine kinase |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 57987.83 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P06239 |
|---|
| Residue: | 509 |
|---|
| Sequence: | MGCGCSSHPEDDWMENIDVCENCHYPIVPLDGKGTLLIRNGSEVRDPLVTYEGSNPPASP
LQDNLVIALHSYEPSHDGDLGFEKGEQLRILEQSGEWWKAQSLTTGQEGFIPFNFVAKAN
SLEPEPWFFKNLSRKDAERQLLAPGNTHGSFLIRESESTAGSFSLSVRDFDQNQGEVVKH
YKIRNLDNGGFYISPRITFPGLHELVRHYTNASDGLCTRLSRPCQTQKPQKPWWEDEWEV
PRETLKLVERLGAGQFGEVWMGYYNGHTKVAVKSLKQGSMSPDAFLAEANLMKQLQHQRL
VRLYAVVTQEPIYIITEYMENGSLVDFLKTPSGIKLTINKLLDMAAQIAEGMAFIEERNY
IHRDLRAANILVSDTLSCKIADFGLARLIEDNEYTAREGAKFPIKWTAPEAINYGTFTIK
SDVWSFGILLTEIVTHGRIPYPGMTNPEVIQNLERGYRMVRPDNCPEELYQLMRLCWKER
PEDRPTFDYLRSVLEDFFTATEGQYQPQP
|
|
|
|---|
| BDBM50186560 |
|---|
| n/a |
|---|
| Name | BDBM50186560 |
|---|
| Synonyms: | 1-(6-(4-(2-(diethylamino)ethoxy)phenylamino)pyrimidin-4-yl)-3-(5-hydroxy-2-methylphenyl)-1-methylurea | CHEMBL206873 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H32N6O3 |
|---|
| Mol. Mass. | 464.56 |
|---|
| SMILES | CCN(CC)CCOc1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2cc(O)ccc2C)cc1 |
|---|
| Structure | 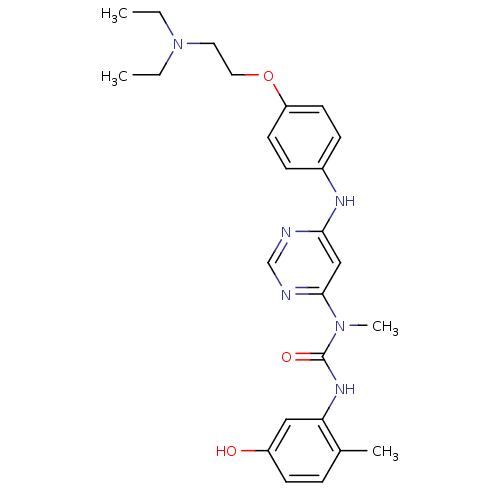
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Maier, JA; Brugel, TA; Sabat, M; Golebiowski, A; Laufersweiler, MJ; VanRens, JC; Hopkins, CR; De, B; Hsieh, LC; Brown, KK; Easwaran, V; Janusz, MJ Development of N-4,6-pyrimidine-N-alkyl-N'-phenyl ureas as orally active inhibitors of lymphocyte specific tyrosine kinase. Bioorg Med Chem Lett16:3646-50 (2006) [PubMed] Article
Maier, JA; Brugel, TA; Sabat, M; Golebiowski, A; Laufersweiler, MJ; VanRens, JC; Hopkins, CR; De, B; Hsieh, LC; Brown, KK; Easwaran, V; Janusz, MJ Development of N-4,6-pyrimidine-N-alkyl-N'-phenyl ureas as orally active inhibitors of lymphocyte specific tyrosine kinase. Bioorg Med Chem Lett16:3646-50 (2006) [PubMed] Article