 Found 496 hits with Last Name = 'laufersweiler' and Initial = 'mj'
Found 496 hits with Last Name = 'laufersweiler' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50474810
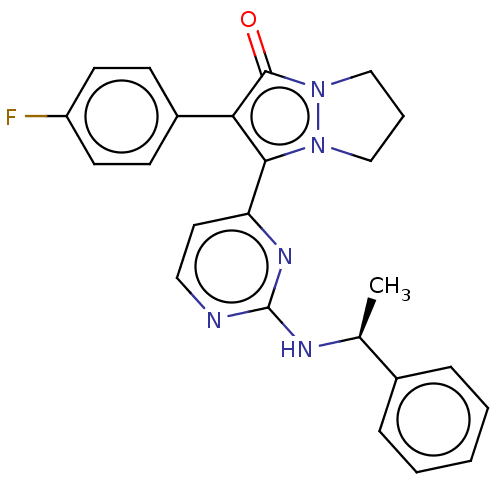
(CHEMBL92082)Show SMILES C[C@H](Nc1nccc(n1)-c1c(-c2ccc(F)cc2)c(=O)n2CCCn12)c1ccccc1 Show InChI InChI=1S/C24H22FN5O/c1-16(17-6-3-2-4-7-17)27-24-26-13-12-20(28-24)22-21(18-8-10-19(25)11-9-18)23(31)30-15-5-14-29(22)30/h2-4,6-13,16H,5,14-15H2,1H3,(H,26,27,28)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of LPS-stimulated p38-related TNF-alpha production in human peripheral blood mononuclear cells (PBMC) |
J Med Chem 47: 2724-7 (2004)
Article DOI: 10.1021/jm049968m
BindingDB Entry DOI: 10.7270/Q2ZC85NQ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101725

(CHEMBL300771 | [4-(Acetyl-benzyl-amino)-cyclohexyl...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@H](C1CCC(CC1)N(Cc1ccccc1)C(C)=O)C(O)=O |wD:18.19,(17.8,3.17,;16.46,3.94,;15.13,3.17,;15.14,1.61,;13.81,.84,;12.48,1.61,;12.47,3.14,;13.79,3.92,;11.15,.83,;11.15,-.71,;9.81,-1.48,;8.48,-.71,;8.48,.83,;9.81,1.6,;7.15,-1.48,;8.24,-2.57,;6.05,-2.57,;5.81,-.71,;4.46,-1.48,;4.48,-3.02,;5.81,-3.78,;5.79,-5.32,;4.46,-6.09,;3.13,-5.32,;3.13,-3.79,;4.46,-7.63,;5.79,-8.41,;5.79,-9.95,;7.12,-10.71,;7.12,-12.23,;5.79,-13.02,;4.44,-12.23,;4.44,-10.71,;3.13,-8.4,;3.13,-9.94,;1.8,-7.63,;3.13,-.71,;1.8,-1.48,;3.13,.83,)| Show InChI InChI=1S/C30H34N2O6S/c1-21(33)32(20-22-6-4-3-5-7-22)26-14-8-25(9-15-26)29(30(34)35)31-39(36,37)28-18-12-24(13-19-28)23-10-16-27(38-2)17-11-23/h3-7,10-13,16-19,25-26,29,31H,8-9,14-15,20H2,1-2H3,(H,34,35)/t25?,26?,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50474810

(CHEMBL92082)Show SMILES C[C@H](Nc1nccc(n1)-c1c(-c2ccc(F)cc2)c(=O)n2CCCn12)c1ccccc1 Show InChI InChI=1S/C24H22FN5O/c1-16(17-6-3-2-4-7-17)27-24-26-13-12-20(28-24)22-21(18-8-10-19(25)11-9-18)23(31)30-15-5-14-29(22)30/h2-4,6-13,16H,5,14-15H2,1H3,(H,26,27,28)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of LPS-stimulated p38-related IL1-beta production in human peripheral blood mononuclear cells (PBMC) |
J Med Chem 47: 2724-7 (2004)
Article DOI: 10.1021/jm049968m
BindingDB Entry DOI: 10.7270/Q2ZC85NQ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50101725

(CHEMBL300771 | [4-(Acetyl-benzyl-amino)-cyclohexyl...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@H](C1CCC(CC1)N(Cc1ccccc1)C(C)=O)C(O)=O |wD:18.19,(17.8,3.17,;16.46,3.94,;15.13,3.17,;15.14,1.61,;13.81,.84,;12.48,1.61,;12.47,3.14,;13.79,3.92,;11.15,.83,;11.15,-.71,;9.81,-1.48,;8.48,-.71,;8.48,.83,;9.81,1.6,;7.15,-1.48,;8.24,-2.57,;6.05,-2.57,;5.81,-.71,;4.46,-1.48,;4.48,-3.02,;5.81,-3.78,;5.79,-5.32,;4.46,-6.09,;3.13,-5.32,;3.13,-3.79,;4.46,-7.63,;5.79,-8.41,;5.79,-9.95,;7.12,-10.71,;7.12,-12.23,;5.79,-13.02,;4.44,-12.23,;4.44,-10.71,;3.13,-8.4,;3.13,-9.94,;1.8,-7.63,;3.13,-.71,;1.8,-1.48,;3.13,.83,)| Show InChI InChI=1S/C30H34N2O6S/c1-21(33)32(20-22-6-4-3-5-7-22)26-14-8-25(9-15-26)29(30(34)35)31-39(36,37)28-18-12-24(13-19-28)23-10-16-27(38-2)17-11-23/h3-7,10-13,16-19,25-26,29,31H,8-9,14-15,20H2,1-2H3,(H,34,35)/t25?,26?,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50101729

(((S)-4'-Methoxy-biphenyl-4-sulfonylamino)-[4-(2-ox...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@H](C1CCC(CC1)N1CCNC1=O)C(O)=O |wD:18.19,(18.85,2.12,;17.51,2.89,;16.18,2.12,;14.84,2.86,;13.52,2.09,;13.53,.55,;14.86,-.22,;16.19,.55,;12.2,-.22,;12.2,-1.76,;10.86,-2.53,;9.53,-1.76,;9.53,-.22,;10.86,.55,;8.2,-2.53,;7.1,-3.62,;9.29,-3.62,;6.86,-1.76,;5.51,-2.53,;5.53,-4.07,;6.86,-4.84,;6.84,-6.38,;5.51,-7.15,;4.18,-6.38,;4.18,-4.84,;5.51,-8.69,;6.77,-9.6,;6.28,-11.07,;4.74,-11.04,;4.28,-9.6,;2.81,-9.11,;4.18,-1.76,;2.85,-2.53,;4.18,-.22,)| Show InChI InChI=1S/C24H29N3O6S/c1-33-20-10-4-16(5-11-20)17-6-12-21(13-7-17)34(31,32)26-22(23(28)29)18-2-8-19(9-3-18)27-15-14-25-24(27)30/h4-7,10-13,18-19,22,26H,2-3,8-9,14-15H2,1H3,(H,25,30)(H,28,29)/t18?,19?,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101729

(((S)-4'-Methoxy-biphenyl-4-sulfonylamino)-[4-(2-ox...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@H](C1CCC(CC1)N1CCNC1=O)C(O)=O |wD:18.19,(18.85,2.12,;17.51,2.89,;16.18,2.12,;14.84,2.86,;13.52,2.09,;13.53,.55,;14.86,-.22,;16.19,.55,;12.2,-.22,;12.2,-1.76,;10.86,-2.53,;9.53,-1.76,;9.53,-.22,;10.86,.55,;8.2,-2.53,;7.1,-3.62,;9.29,-3.62,;6.86,-1.76,;5.51,-2.53,;5.53,-4.07,;6.86,-4.84,;6.84,-6.38,;5.51,-7.15,;4.18,-6.38,;4.18,-4.84,;5.51,-8.69,;6.77,-9.6,;6.28,-11.07,;4.74,-11.04,;4.28,-9.6,;2.81,-9.11,;4.18,-1.76,;2.85,-2.53,;4.18,-.22,)| Show InChI InChI=1S/C24H29N3O6S/c1-33-20-10-4-16(5-11-20)17-6-12-21(13-7-17)34(31,32)26-22(23(28)29)18-2-8-19(9-3-18)27-15-14-25-24(27)30/h4-7,10-13,18-19,22,26H,2-3,8-9,14-15H2,1H3,(H,25,30)(H,28,29)/t18?,19?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50188343
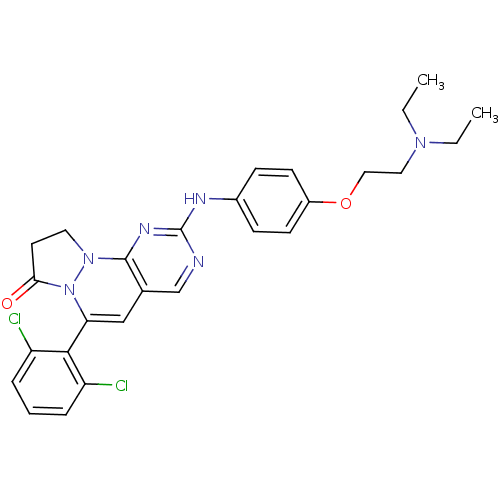
(4-(2,6-dichloro-phenyl)-8-[4-(2-diethylamino-ethox...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3C=C(N4N(CCC4=O)c3n2)c2c(Cl)cccc2Cl)cc1 |c:17| Show InChI InChI=1S/C27H28Cl2N6O2/c1-3-33(4-2)14-15-37-20-10-8-19(9-11-20)31-27-30-17-18-16-23(25-21(28)6-5-7-22(25)29)35-24(36)12-13-34(35)26(18)32-27/h5-11,16-17H,3-4,12-15H2,1-2H3,(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Lck in presence of 10 mM ATP |
Bioorg Med Chem Lett 16: 4257-61 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.072
BindingDB Entry DOI: 10.7270/Q22V2FQD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50474826

(CHEMBL330282)Show SMILES CC[C@H](C)Nc1nccc(n1)-c1c(-c2ccc(F)cc2)c(=O)n2CCCn12 Show InChI InChI=1S/C20H22FN5O/c1-3-13(2)23-20-22-10-9-16(24-20)18-17(14-5-7-15(21)8-6-14)19(27)26-12-4-11-25(18)26/h5-10,13H,3-4,11-12H2,1-2H3,(H,22,23,24)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against LPS-stimulated TNF-alpha production in human monocytic cells (THP-1) |
J Med Chem 47: 2724-7 (2004)
Article DOI: 10.1021/jm049968m
BindingDB Entry DOI: 10.7270/Q2ZC85NQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50186560
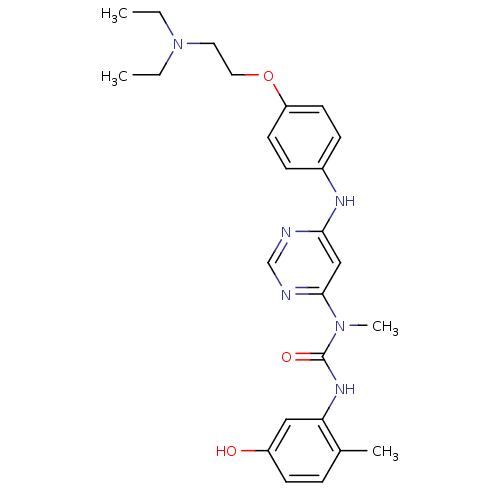
(1-(6-(4-(2-(diethylamino)ethoxy)phenylamino)pyrimi...)Show SMILES CCN(CC)CCOc1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2cc(O)ccc2C)cc1 Show InChI InChI=1S/C25H32N6O3/c1-5-31(6-2)13-14-34-21-11-8-19(9-12-21)28-23-16-24(27-17-26-23)30(4)25(33)29-22-15-20(32)10-7-18(22)3/h7-12,15-17,32H,5-6,13-14H2,1-4H3,(H,29,33)(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 16: 3646-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.072
BindingDB Entry DOI: 10.7270/Q2GF0T45 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50186557
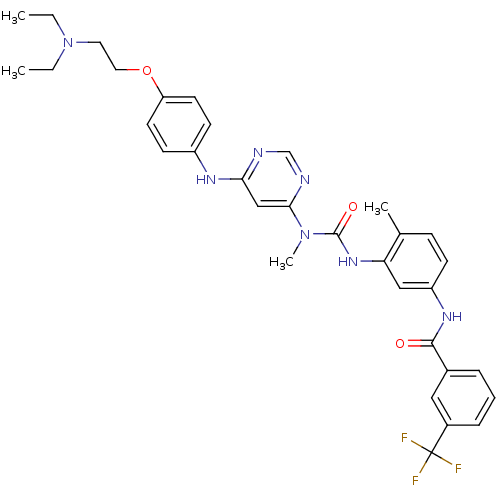
(1-(6-(4-(2-(diethylamino)ethoxy)phenylamino)pyrimi...)Show SMILES CCN(CC)CCOc1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2cc(NC(=O)c3cccc(c3)C(F)(F)F)ccc2C)cc1 Show InChI InChI=1S/C33H36F3N7O3/c1-5-43(6-2)16-17-46-27-14-12-25(13-15-27)39-29-20-30(38-21-37-29)42(4)32(45)41-28-19-26(11-10-22(28)3)40-31(44)23-8-7-9-24(18-23)33(34,35)36/h7-15,18-21H,5-6,16-17H2,1-4H3,(H,40,44)(H,41,45)(H,37,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 16: 3646-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.072
BindingDB Entry DOI: 10.7270/Q2GF0T45 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50188343

(4-(2,6-dichloro-phenyl)-8-[4-(2-diethylamino-ethox...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3C=C(N4N(CCC4=O)c3n2)c2c(Cl)cccc2Cl)cc1 |c:17| Show InChI InChI=1S/C27H28Cl2N6O2/c1-3-33(4-2)14-15-37-20-10-8-19(9-11-20)31-27-30-17-18-16-23(25-21(28)6-5-7-22(25)29)35-24(36)12-13-34(35)26(18)32-27/h5-11,16-17H,3-4,12-15H2,1-2H3,(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src in presence of 10 mM ATP |
Bioorg Med Chem Lett 16: 4257-61 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.072
BindingDB Entry DOI: 10.7270/Q22V2FQD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50188342
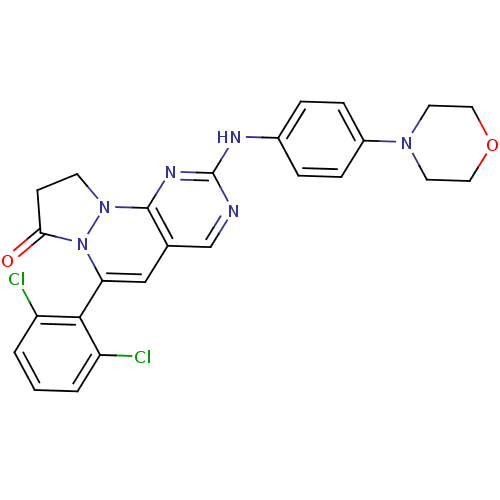
(4-(2,6-dichloro-phenyl)-8-(4-morpholin-4-yl-phenyl...)Show SMILES Clc1cccc(Cl)c1C1=Cc2cnc(Nc3ccc(cc3)N3CCOCC3)nc2N2CCC(=O)N12 |t:9| Show InChI InChI=1S/C25H22Cl2N6O2/c26-19-2-1-3-20(27)23(19)21-14-16-15-28-25(30-24(16)32-9-8-22(34)33(21)32)29-17-4-6-18(7-5-17)31-10-12-35-13-11-31/h1-7,14-15H,8-13H2,(H,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Lck in presence of 10 mM ATP |
Bioorg Med Chem Lett 16: 4257-61 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.072
BindingDB Entry DOI: 10.7270/Q22V2FQD |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101730
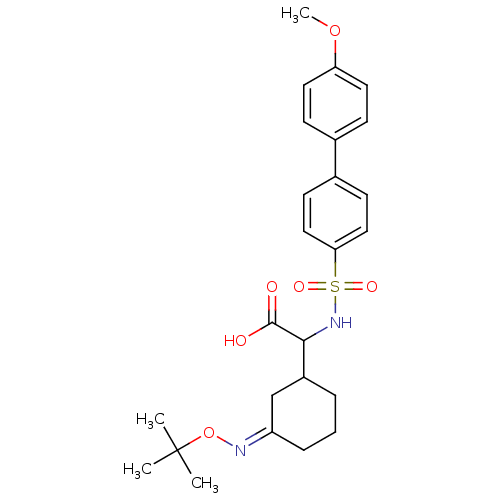
(CHEMBL59472 | {3-[(Z)-tert-Butoxyimino]-cyclohexyl...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCC\C(C1)=N\OC(C)(C)C)C(O)=O Show InChI InChI=1S/C25H32N2O6S/c1-25(2,3)33-26-20-7-5-6-19(16-20)23(24(28)29)27-34(30,31)22-14-10-18(11-15-22)17-8-12-21(32-4)13-9-17/h8-15,19,23,27H,5-7,16H2,1-4H3,(H,28,29)/b26-20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50195345

(3-(2-(1H-benzo[d]imidazol-1-yl)-6-(4-methylpiperaz...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(O)ccc2C)nc(n1)-n1cnc2ccccc12 Show InChI InChI=1S/C23H25N7O/c1-16-7-8-17(31)13-19(16)25-21-14-22(29-11-9-28(2)10-12-29)27-23(26-21)30-15-24-18-5-3-4-6-20(18)30/h3-8,13-15,31H,9-12H2,1-2H3,(H,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Lck by ProFlour assay |
Bioorg Med Chem Lett 16: 5973-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.132
BindingDB Entry DOI: 10.7270/Q2028R5P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50195351

(1-(3-(2,5-dimethylbenzyl)-5-(3-cyclohexylpropyl)ph...)Show SMILES Cc1ccc(O)cc1Nc1cc(OCCN2CCOCC2)nc(n1)-n1cnc2ccccc12 Show InChI InChI=1S/C24H26N6O3/c1-17-6-7-18(31)14-20(17)26-22-15-23(33-13-10-29-8-11-32-12-9-29)28-24(27-22)30-16-25-19-4-2-3-5-21(19)30/h2-7,14-16,31H,8-13H2,1H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Lck by ProFlour assay |
Bioorg Med Chem Lett 16: 5973-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.132
BindingDB Entry DOI: 10.7270/Q2028R5P |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50474810

(CHEMBL92082)Show SMILES C[C@H](Nc1nccc(n1)-c1c(-c2ccc(F)cc2)c(=O)n2CCCn12)c1ccccc1 Show InChI InChI=1S/C24H22FN5O/c1-16(17-6-3-2-4-7-17)27-24-26-13-12-20(28-24)22-21(18-8-10-19(25)11-9-18)23(31)30-15-5-14-29(22)30/h2-4,6-13,16H,5,14-15H2,1H3,(H,26,27,28)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against LPS-stimulated TNF-alpha production in human monocytic cells (THP-1) |
J Med Chem 47: 2724-7 (2004)
Article DOI: 10.1021/jm049968m
BindingDB Entry DOI: 10.7270/Q2ZC85NQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50195334
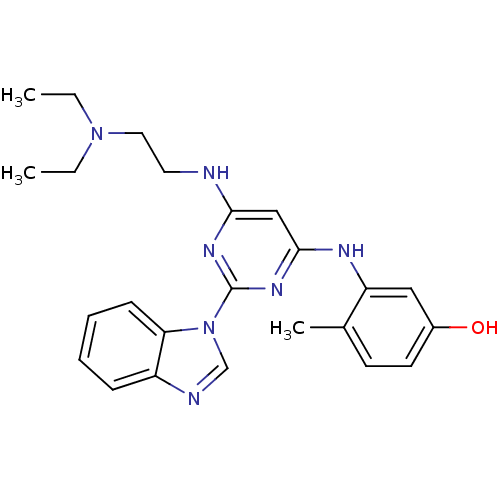
(3-(2-(1H-benzo[d]imidazol-1-yl)-6-(2-(diethylamino...)Show SMILES CCN(CC)CCNc1cc(Nc2cc(O)ccc2C)nc(n1)-n1cnc2ccccc12 Show InChI InChI=1S/C24H29N7O/c1-4-30(5-2)13-12-25-22-15-23(27-20-14-18(32)11-10-17(20)3)29-24(28-22)31-16-26-19-8-6-7-9-21(19)31/h6-11,14-16,32H,4-5,12-13H2,1-3H3,(H2,25,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Lck by ProFlour assay |
Bioorg Med Chem Lett 16: 5973-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.132
BindingDB Entry DOI: 10.7270/Q2028R5P |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50474831
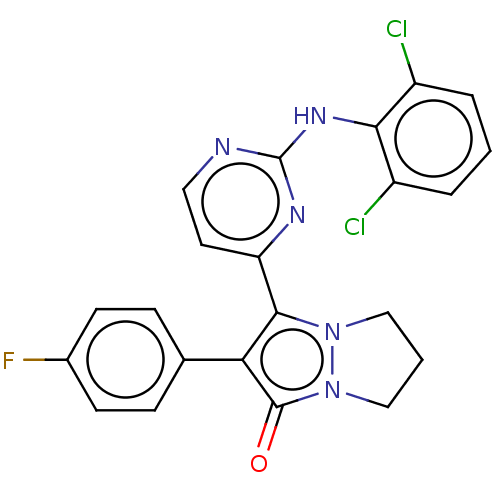
(CHEMBL92415)Show SMILES Fc1ccc(cc1)-c1c(-c2ccnc(Nc3c(Cl)cccc3Cl)n2)n2CCCn2c1=O Show InChI InChI=1S/C22H16Cl2FN5O/c23-15-3-1-4-16(24)19(15)28-22-26-10-9-17(27-22)20-18(13-5-7-14(25)8-6-13)21(31)30-12-2-11-29(20)30/h1,3-10H,2,11-12H2,(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against LPS-stimulated TNF-alpha production in human monocytic cells (THP-1) |
J Med Chem 47: 2724-7 (2004)
Article DOI: 10.1021/jm049968m
BindingDB Entry DOI: 10.7270/Q2ZC85NQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50186535
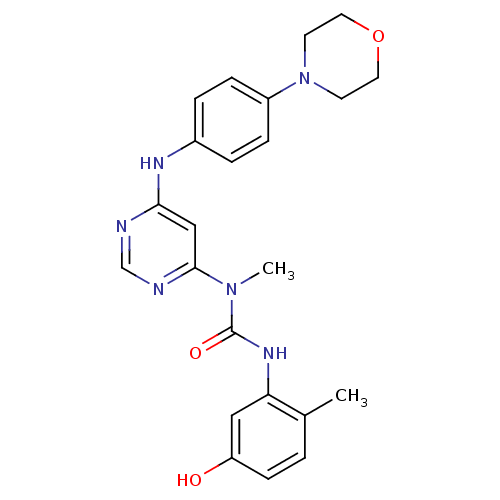
(3-(5-hydroxy-2-methylphenyl)-1-methyl-1-(6-(4-morp...)Show SMILES CN(C(=O)Nc1cc(O)ccc1C)c1cc(Nc2ccc(cc2)N2CCOCC2)ncn1 Show InChI InChI=1S/C23H26N6O3/c1-16-3-8-19(30)13-20(16)27-23(31)28(2)22-14-21(24-15-25-22)26-17-4-6-18(7-5-17)29-9-11-32-12-10-29/h3-8,13-15,30H,9-12H2,1-2H3,(H,27,31)(H,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 16: 3646-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.072
BindingDB Entry DOI: 10.7270/Q2GF0T45 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50186566
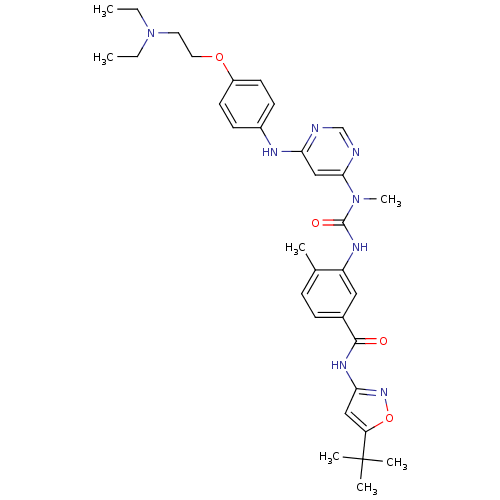
(3-(5-((5-tert-butylisoxazol-3-yl)carbamoyl)-2-meth...)Show SMILES CCN(CC)CCOc1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2cc(ccc2C)C(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C33H42N8O4/c1-8-41(9-2)16-17-44-25-14-12-24(13-15-25)36-28-20-30(35-21-34-28)40(7)32(43)37-26-18-23(11-10-22(26)3)31(42)38-29-19-27(45-39-29)33(4,5)6/h10-15,18-21H,8-9,16-17H2,1-7H3,(H,37,43)(H,34,35,36)(H,38,39,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 16: 3646-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.072
BindingDB Entry DOI: 10.7270/Q2GF0T45 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50195340
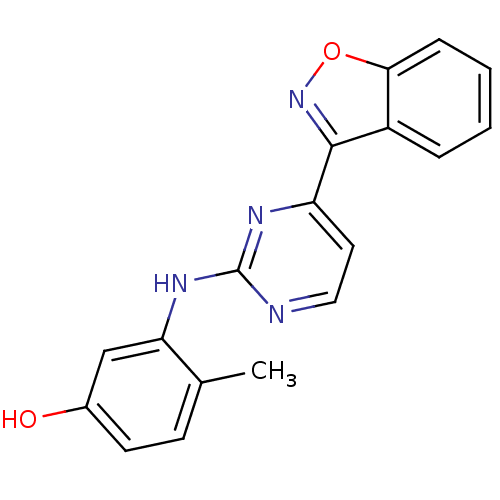
(3-(4-(benzo[d]isoxazol-3-yl)pyrimidin-2-ylamino)-4...)Show InChI InChI=1S/C18H14N4O2/c1-11-6-7-12(23)10-15(11)21-18-19-9-8-14(20-18)17-13-4-2-3-5-16(13)24-22-17/h2-10,23H,1H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Lck by ProFlour assay |
Bioorg Med Chem Lett 16: 5973-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.132
BindingDB Entry DOI: 10.7270/Q2028R5P |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101717
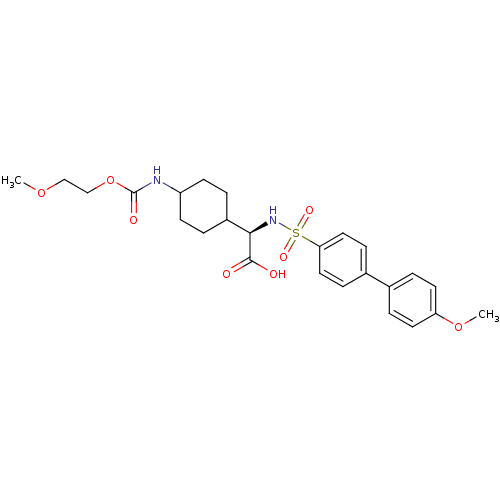
(((S)-4'-Methoxy-biphenyl-4-sulfonylamino)-[4-(2-me...)Show SMILES COCCOC(=O)NC1CCC(CC1)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O |wD:14.15,(12.14,-8.5,;10.81,-9.24,;9.48,-8.47,;8.12,-9.24,;6.79,-8.47,;5.46,-9.24,;5.46,-10.79,;4.12,-8.47,;4.12,-6.93,;5.46,-6.16,;5.48,-4.61,;4.15,-3.84,;2.79,-4.61,;2.79,-6.16,;4.12,-2.3,;5.48,-1.53,;6.81,-2.3,;5.71,-3.4,;7.91,-3.4,;8.14,-1.53,;9.48,-2.3,;10.83,-1.53,;10.83,.02,;9.48,.79,;8.14,.02,;12.17,.79,;12.14,2.33,;13.48,3.1,;14.81,2.35,;16.14,3.13,;17.5,2.35,;14.83,.79,;13.5,.02,;2.79,-1.53,;1.46,-2.3,;2.79,.02,)| Show InChI InChI=1S/C25H32N2O8S/c1-33-15-16-35-25(30)26-20-9-3-19(4-10-20)23(24(28)29)27-36(31,32)22-13-7-18(8-14-22)17-5-11-21(34-2)12-6-17/h5-8,11-14,19-20,23,27H,3-4,9-10,15-16H2,1-2H3,(H,26,30)(H,28,29)/t19?,20?,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101716
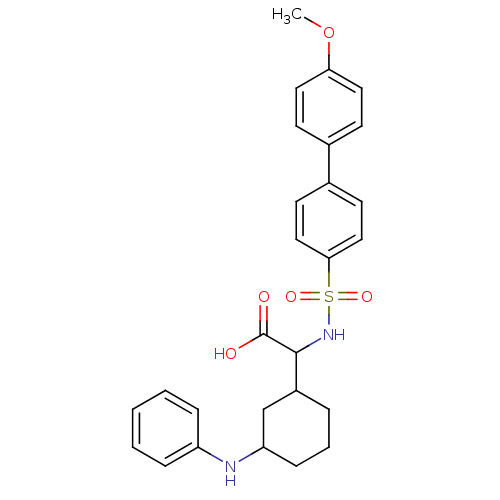
((4'-Methoxy-biphenyl-4-sulfonylamino)-(3-phenylami...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCCC(C1)Nc1ccccc1)C(O)=O Show InChI InChI=1S/C27H30N2O5S/c1-34-24-14-10-19(11-15-24)20-12-16-25(17-13-20)35(32,33)29-26(27(30)31)21-6-5-9-23(18-21)28-22-7-3-2-4-8-22/h2-4,7-8,10-17,21,23,26,28-29H,5-6,9,18H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50474829

(CHEMBL433211)Show SMILES Fc1ccc(cc1)-c1c(-c2ccnc(Nc3c(F)cccc3F)n2)n2CCCn2c1=O Show InChI InChI=1S/C22H16F3N5O/c23-14-7-5-13(6-8-14)18-20(29-11-2-12-30(29)21(18)31)17-9-10-26-22(27-17)28-19-15(24)3-1-4-16(19)25/h1,3-10H,2,11-12H2,(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against LPS-stimulated TNF-alpha production in human monocytic cells (THP-1) |
J Med Chem 47: 2724-7 (2004)
Article DOI: 10.1021/jm049968m
BindingDB Entry DOI: 10.7270/Q2ZC85NQ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50098298
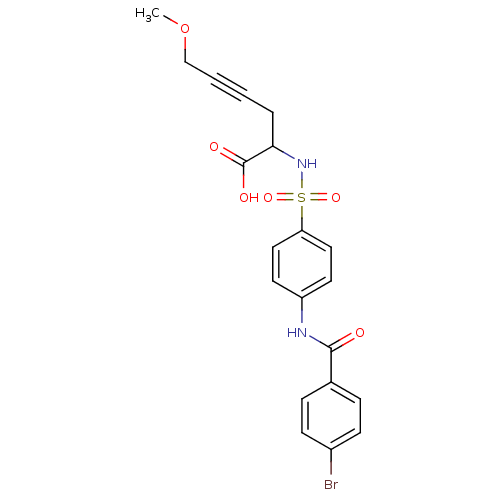
(2-[4-(4-Bromo-benzoylamino)-benzenesulfonylamino]-...)Show SMILES COCC#CCC(NS(=O)(=O)c1ccc(NC(=O)c2ccc(Br)cc2)cc1)C(O)=O Show InChI InChI=1S/C20H19BrN2O6S/c1-29-13-3-2-4-18(20(25)26)23-30(27,28)17-11-9-16(10-12-17)22-19(24)14-5-7-15(21)8-6-14/h5-12,18,23H,4,13H2,1H3,(H,22,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase-2 (MMP-2) in quenched fluorescence assay at pH 7.4 |
J Med Chem 44: 1060-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RN374T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50186556

(3-(2,6-dichlorophenyl)-1-methyl-1-(6-(4-(4-methylp...)Show SMILES CN(C(=O)Nc1c(Cl)cccc1Cl)c1cc(Nc2ccc(cc2)N2CCN(C)CC2)ncn1 Show InChI InChI=1S/C23H25Cl2N7O/c1-30-10-12-32(13-11-30)17-8-6-16(7-9-17)28-20-14-21(27-15-26-20)31(2)23(33)29-22-18(24)4-3-5-19(22)25/h3-9,14-15H,10-13H2,1-2H3,(H,29,33)(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 16: 3646-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.072
BindingDB Entry DOI: 10.7270/Q2GF0T45 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
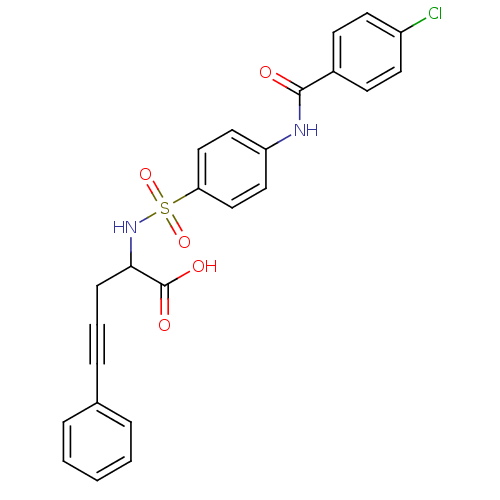
(Homo sapiens (Human)) | BDBM50098260
(2-[4-(4-Chloro-benzoylamino)-benzenesulfonylamino]...)Show SMILES OC(=O)C(CC#Cc1ccccc1)NS(=O)(=O)c1ccc(NC(=O)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C24H19ClN2O5S/c25-19-11-9-18(10-12-19)23(28)26-20-13-15-21(16-14-20)33(31,32)27-22(24(29)30)8-4-7-17-5-2-1-3-6-17/h1-3,5-6,9-16,22,27H,8H2,(H,26,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase-2 (MMP-2) in quenched fluorescence assay at pH 7.4 |
J Med Chem 44: 1060-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RN374T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
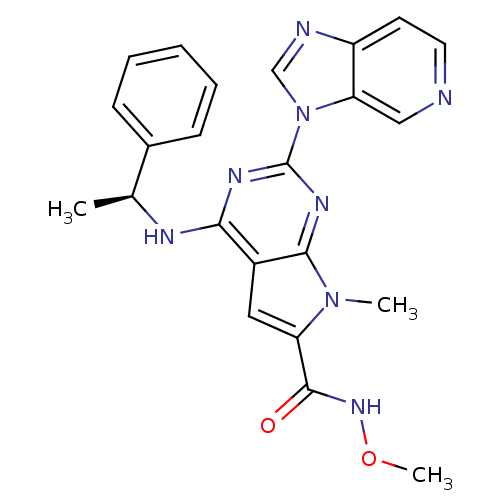
(Homo sapiens (Human)) | BDBM50201514
(2-(3H-imidazo[4,5-c]pyridin-3-yl)-N-methoxy-7-meth...)Show SMILES CONC(=O)c1cc2c(N[C@@H](C)c3ccccc3)nc(nc2n1C)-n1cnc2ccncc12 Show InChI InChI=1S/C23H22N8O2/c1-14(15-7-5-4-6-8-15)26-20-16-11-18(22(32)29-33-3)30(2)21(16)28-23(27-20)31-13-25-17-9-10-24-12-19(17)31/h4-14H,1-3H3,(H,29,32)(H,26,27,28)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by kinase-Glo luminescent assay |
Bioorg Med Chem Lett 17: 1250-3 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.018
BindingDB Entry DOI: 10.7270/Q2H70GMJ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101712
(CHEMBL300375 | {3-[Benzyl-(2-methoxy-ethoxycarbony...)Show SMILES COCCOC(=O)N(Cc1ccccc1)C1CCCC(C1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C32H38N2O8S/c1-40-19-20-42-32(37)34(22-23-7-4-3-5-8-23)27-10-6-9-26(21-27)30(31(35)36)33-43(38,39)29-17-13-25(14-18-29)24-11-15-28(41-2)16-12-24/h3-5,7-8,11-18,26-27,30,33H,6,9-10,19-22H2,1-2H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50098275
(2-(4'-Methylsulfanyl-biphenyl-4-sulfonylamino)-5-p...)Show SMILES CSc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(CC#Cc1ccccc1)C(O)=O Show InChI InChI=1S/C24H21NO4S2/c1-30-21-14-10-19(11-15-21)20-12-16-22(17-13-20)31(28,29)25-23(24(26)27)9-5-8-18-6-3-2-4-7-18/h2-4,6-7,10-17,23,25H,9H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase-2 (MMP-2) in quenched fluorescence assay at pH 7.4 |
J Med Chem 44: 1060-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RN374T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50195354
(CHEMBL220924 | N1-(4-(benzo[d]isoxazol-3-yl)pyrimi...)Show InChI InChI=1S/C18H15N5O/c1-11-6-7-12(19)10-15(11)22-18-20-9-8-14(21-18)17-13-4-2-3-5-16(13)24-23-17/h2-10H,19H2,1H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Lck by ProFlour assay |
Bioorg Med Chem Lett 16: 5973-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.132
BindingDB Entry DOI: 10.7270/Q2028R5P |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101723

((1,5-Dioxa-spiro[5.5]undec-9-yl)-((S)-4'-methoxy-b...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@H](C1CCC2(CC1)OCCCO2)C(O)=O Show InChI InChI=1S/C24H29NO7S/c1-30-20-7-3-17(4-8-20)18-5-9-21(10-6-18)33(28,29)25-22(23(26)27)19-11-13-24(14-12-19)31-15-2-16-32-24/h3-10,19,22,25H,2,11-16H2,1H3,(H,26,27)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50101730

(CHEMBL59472 | {3-[(Z)-tert-Butoxyimino]-cyclohexyl...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCC\C(C1)=N\OC(C)(C)C)C(O)=O Show InChI InChI=1S/C25H32N2O6S/c1-25(2,3)33-26-20-7-5-6-19(16-20)23(24(28)29)27-34(30,31)22-14-10-18(11-15-22)17-8-12-21(32-4)13-9-17/h8-15,19,23,27H,5-7,16H2,1-4H3,(H,28,29)/b26-20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101715

((4-Benzylamino-cyclohexyl)-((S)-4'-methoxy-bipheny...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@H](C1CCC(CC1)NCc1ccccc1)C(O)=O |wD:18.19,(18.21,3.1,;16.87,3.89,;15.54,3.1,;14.19,3.87,;12.88,3.08,;12.89,1.56,;14.22,.79,;15.55,1.56,;11.56,.77,;11.56,-.77,;10.22,-1.54,;8.89,-.77,;8.89,.77,;10.22,1.54,;7.56,-1.54,;8.64,-2.64,;6.46,-2.64,;6.21,-.77,;4.88,-1.54,;4.88,-3.08,;6.21,-3.83,;6.21,-5.37,;4.88,-6.14,;3.55,-5.37,;3.55,-3.85,;4.88,-7.68,;6.21,-8.47,;6.21,-10.01,;7.54,-10.76,;7.54,-12.3,;6.19,-13.07,;4.85,-12.3,;4.86,-10.76,;3.55,-.77,;2.22,-1.54,;3.55,.77,)| Show InChI InChI=1S/C28H32N2O5S/c1-35-25-15-9-21(10-16-25)22-11-17-26(18-12-22)36(33,34)30-27(28(31)32)23-7-13-24(14-8-23)29-19-20-5-3-2-4-6-20/h2-6,9-12,15-18,23-24,27,29-30H,7-8,13-14,19H2,1H3,(H,31,32)/t23?,24?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50474810

(CHEMBL92082)Show SMILES C[C@H](Nc1nccc(n1)-c1c(-c2ccc(F)cc2)c(=O)n2CCCn12)c1ccccc1 Show InChI InChI=1S/C24H22FN5O/c1-16(17-6-3-2-4-7-17)27-24-26-13-12-20(28-24)22-21(18-8-10-19(25)11-9-18)23(31)30-15-5-14-29(22)30/h2-4,6-13,16H,5,14-15H2,1H3,(H,26,27,28)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Mitogen-activated protein kinase p38 |
J Med Chem 47: 2724-7 (2004)
Article DOI: 10.1021/jm049968m
BindingDB Entry DOI: 10.7270/Q2ZC85NQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50474817

(CHEMBL93281)Show SMILES C[C@H](Nc1nccc(n1)-c1c(-c2ccc(F)cc2)c(=O)n2CCCn12)C(C)(C)O Show InChI InChI=1S/C21H24FN5O2/c1-13(21(2,3)29)24-20-23-10-9-16(25-20)18-17(14-5-7-15(22)8-6-14)19(28)27-12-4-11-26(18)27/h5-10,13,29H,4,11-12H2,1-3H3,(H,23,24,25)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of LPS-stimulated p38-related TNF-alpha production in human peripheral blood mononuclear cells (PBMC) |
J Med Chem 47: 2724-7 (2004)
Article DOI: 10.1021/jm049968m
BindingDB Entry DOI: 10.7270/Q2ZC85NQ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50098295

(3-Methoxy-2-(4'-methylsulfanyl-biphenyl-4-sulfonyl...)Show SMILES CO[C@H](C#Cc1ccccc1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(SC)cc1)C(O)=O Show InChI InChI=1S/C25H23NO5S2/c1-31-23(17-8-18-6-4-3-5-7-18)24(25(27)28)26-33(29,30)22-15-11-20(12-16-22)19-9-13-21(32-2)14-10-19/h3-7,9-16,23-24,26H,1-2H3,(H,27,28)/t23-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase-2 (MMP-2) in quenched fluorescence assay at pH 7.4 |
J Med Chem 44: 1060-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RN374T |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101714
((4'-Methoxy-biphenyl-4-sulfonylamino)-{3-[(2-metho...)Show SMILES COCCOC(=O)N(C)C1CCCC(C1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C26H34N2O8S/c1-28(26(31)36-16-15-34-2)21-6-4-5-20(17-21)24(25(29)30)27-37(32,33)23-13-9-19(10-14-23)18-7-11-22(35-3)12-8-18/h7-14,20-21,24,27H,4-6,15-17H2,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50098282

(6-Methoxy-2-[4-(4-methoxy-benzoylamino)-benzenesul...)Show SMILES COCC#CCC(NS(=O)(=O)c1ccc(NC(=O)c2ccc(OC)cc2)cc1)C(O)=O Show InChI InChI=1S/C21H22N2O7S/c1-29-14-4-3-5-19(21(25)26)23-31(27,28)18-12-8-16(9-13-18)22-20(24)15-6-10-17(30-2)11-7-15/h6-13,19,23H,5,14H2,1-2H3,(H,22,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase-2 (MMP-2) in quenched fluorescence assay at pH 7.4 |
J Med Chem 44: 1060-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RN374T |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101721
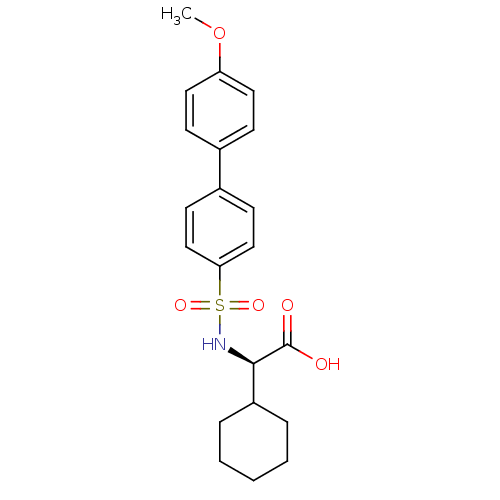
(CHEMBL293019 | Cyclohexyl-((S)-4'-methoxy-biphenyl...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@H](C1CCCCC1)C(O)=O Show InChI InChI=1S/C21H25NO5S/c1-27-18-11-7-15(8-12-18)16-9-13-19(14-10-16)28(25,26)22-20(21(23)24)17-5-3-2-4-6-17/h7-14,17,20,22H,2-6H2,1H3,(H,23,24)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50101716

((4'-Methoxy-biphenyl-4-sulfonylamino)-(3-phenylami...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCCC(C1)Nc1ccccc1)C(O)=O Show InChI InChI=1S/C27H30N2O5S/c1-34-24-14-10-19(11-15-24)20-12-16-25(17-13-20)35(32,33)29-26(27(30)31)21-6-5-9-23(18-21)28-22-7-3-2-4-8-22/h2-4,7-8,10-17,21,23,26,28-29H,5-6,9,18H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50098287
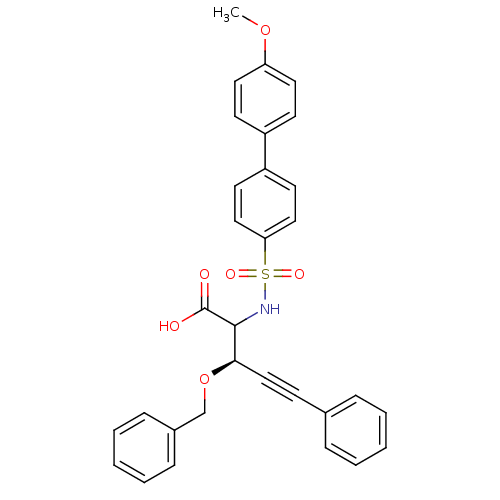
(3-Benzyloxy-2-(4'-methoxy-biphenyl-4-sulfonylamino...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC([C@H](OCc1ccccc1)C#Cc1ccccc1)C(O)=O Show InChI InChI=1S/C31H27NO6S/c1-37-27-17-13-25(14-18-27)26-15-19-28(20-16-26)39(35,36)32-30(31(33)34)29(21-12-23-8-4-2-5-9-23)38-22-24-10-6-3-7-11-24/h2-11,13-20,29-30,32H,22H2,1H3,(H,33,34)/t29-,30?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase-2 (MMP-2) in quenched fluorescence assay at pH 7.4 |
J Med Chem 44: 1060-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RN374T |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50098297
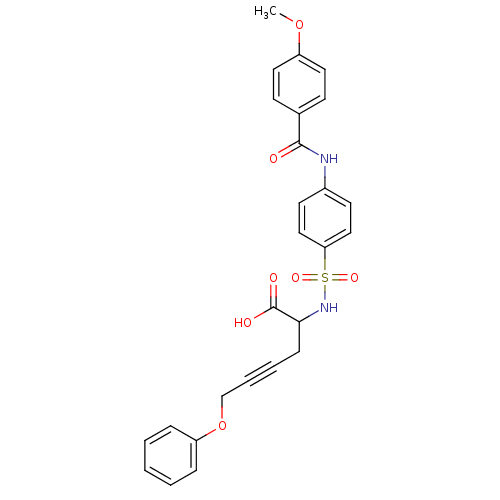
(2-[4-(4-Methoxy-benzoylamino)-benzenesulfonylamino...)Show SMILES COc1ccc(cc1)C(=O)Nc1ccc(cc1)S(=O)(=O)NC(CC#CCOc1ccccc1)C(O)=O Show InChI InChI=1S/C26H24N2O7S/c1-34-21-14-10-19(11-15-21)25(29)27-20-12-16-23(17-13-20)36(32,33)28-24(26(30)31)9-5-6-18-35-22-7-3-2-4-8-22/h2-4,7-8,10-17,24,28H,9,18H2,1H3,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase-2 (MMP-2) in quenched fluorescence assay at pH 7.4 |
J Med Chem 44: 1060-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RN374T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50186554
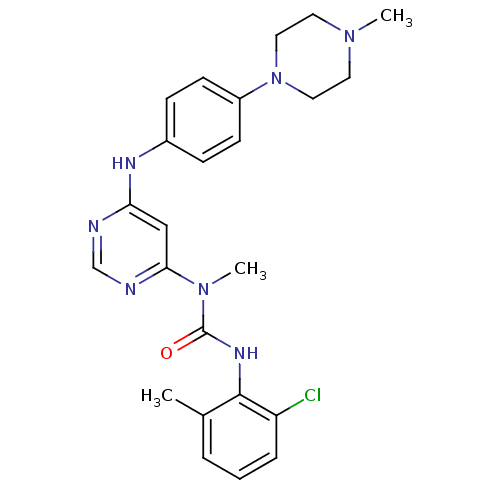
(3-(2-chloro-6-methylphenyl)-1-methyl-1-(6-(4-(4-me...)Show SMILES CN(C(=O)Nc1c(C)cccc1Cl)c1cc(Nc2ccc(cc2)N2CCN(C)CC2)ncn1 Show InChI InChI=1S/C24H28ClN7O/c1-17-5-4-6-20(25)23(17)29-24(33)31(3)22-15-21(26-16-27-22)28-18-7-9-19(10-8-18)32-13-11-30(2)12-14-32/h4-10,15-16H,11-14H2,1-3H3,(H,29,33)(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Lck |
Bioorg Med Chem Lett 16: 3646-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.072
BindingDB Entry DOI: 10.7270/Q2GF0T45 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50098261
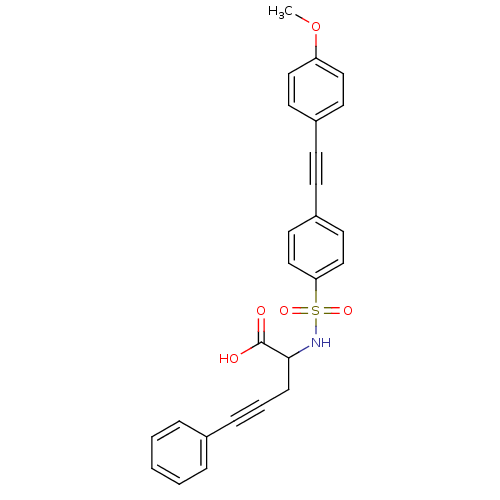
(2-[4-(4-Methoxy-phenylethynyl)-benzenesulfonylamin...)Show SMILES COc1ccc(cc1)C#Cc1ccc(cc1)S(=O)(=O)NC(CC#Cc1ccccc1)C(O)=O Show InChI InChI=1S/C26H21NO5S/c1-32-23-16-12-21(13-17-23)10-11-22-14-18-24(19-15-22)33(30,31)27-25(26(28)29)9-5-8-20-6-3-2-4-7-20/h2-4,6-7,12-19,25,27H,9H2,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase-2 (MMP-2) in quenched fluorescence assay at pH 7.4 |
J Med Chem 44: 1060-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RN374T |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101733
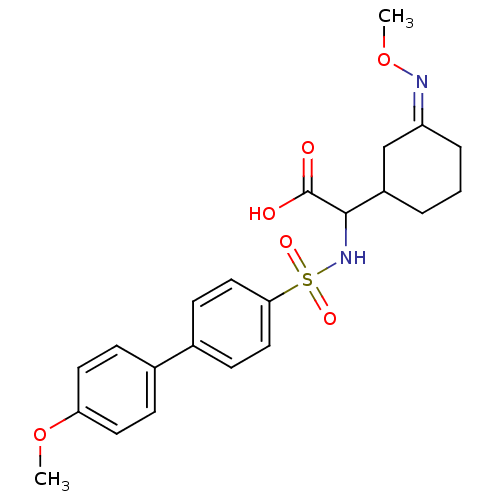
((4'-Methoxy-biphenyl-4-sulfonylamino)-{3-[(Z)-meth...)Show SMILES CO\N=C1\CCCC(C1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C22H26N2O6S/c1-29-19-10-6-15(7-11-19)16-8-12-20(13-9-16)31(27,28)24-21(22(25)26)17-4-3-5-18(14-17)23-30-2/h6-13,17,21,24H,3-5,14H2,1-2H3,(H,25,26)/b23-18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50098268
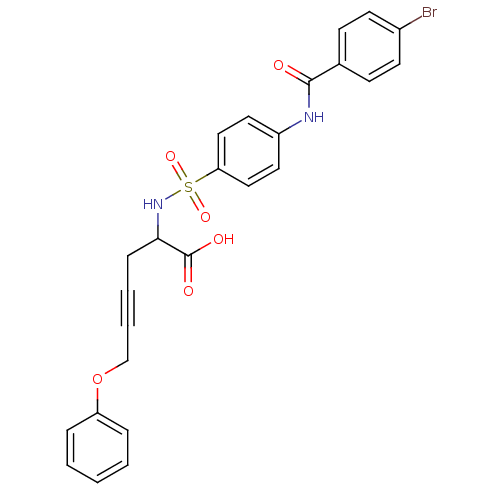
(2-[4-(4-Bromo-benzoylamino)-benzenesulfonylamino]-...)Show SMILES OC(=O)C(CC#CCOc1ccccc1)NS(=O)(=O)c1ccc(NC(=O)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C25H21BrN2O6S/c26-19-11-9-18(10-12-19)24(29)27-20-13-15-22(16-14-20)35(32,33)28-23(25(30)31)8-4-5-17-34-21-6-2-1-3-7-21/h1-3,6-7,9-16,23,28H,8,17H2,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase-2 (MMP-2) in quenched fluorescence assay at pH 7.4 |
J Med Chem 44: 1060-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RN374T |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101720

((1,4-Dioxa-spiro[4.5]dec-7-yl)-(4'-methoxy-bipheny...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCCC2(C1)OCCO2)C(O)=O Show InChI InChI=1S/C23H27NO7S/c1-29-19-8-4-16(5-9-19)17-6-10-20(11-7-17)32(27,28)24-21(22(25)26)18-3-2-12-23(15-18)30-13-14-31-23/h4-11,18,21,24H,2-3,12-15H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50188348
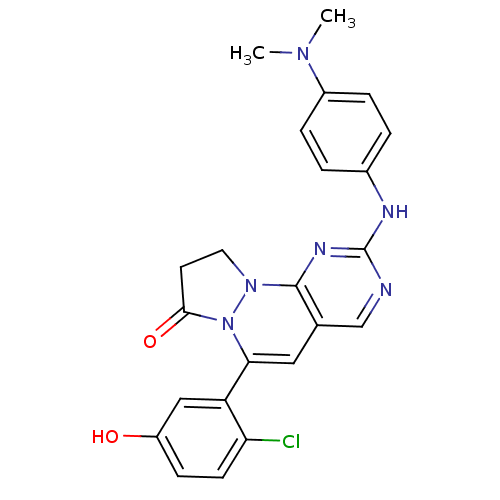
(4-(2-chloro-5-hydroxy-phenyl)-8-(4-dimethylamino-p...)Show SMILES CN(C)c1ccc(Nc2ncc3C=C(N4N(CCC4=O)c3n2)c2cc(O)ccc2Cl)cc1 |c:12| Show InChI InChI=1S/C23H21ClN6O2/c1-28(2)16-5-3-15(4-6-16)26-23-25-13-14-11-20(18-12-17(31)7-8-19(18)24)30-21(32)9-10-29(30)22(14)27-23/h3-8,11-13,31H,9-10H2,1-2H3,(H,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Lck in presence of 10 mM ATP |
Bioorg Med Chem Lett 16: 4257-61 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.072
BindingDB Entry DOI: 10.7270/Q22V2FQD |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50101719

((4'-Methoxy-biphenyl-4-sulfonylamino)-[3-(2-oxo-ox...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCCC(C1)N1CCOC1=O)C(O)=O Show InChI InChI=1S/C24H28N2O7S/c1-32-20-9-5-16(6-10-20)17-7-11-21(12-8-17)34(30,31)25-22(23(27)28)18-3-2-4-19(15-18)26-13-14-33-24(26)29/h5-12,18-19,22,25H,2-4,13-15H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 1975-9 (2001)
BindingDB Entry DOI: 10.7270/Q2S181SV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data