

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Integrase | ||
| Ligand | BDBM50187767 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_378064 (CHEMBL869770) | ||
| IC50 | 350±n/a nM | ||
| Citation |  Jin, H; Cai, RZ; Schacherer, L; Jabri, S; Tsiang, M; Fardis, M; Chen, X; Chen, JM; Kim, CU Design, synthesis, and SAR studies of novel and highly active tri-cyclic HIV integrase inhibitors. Bioorg Med Chem Lett16:3989-92 (2006) [PubMed] Article Jin, H; Cai, RZ; Schacherer, L; Jabri, S; Tsiang, M; Fardis, M; Chen, X; Chen, JM; Kim, CU Design, synthesis, and SAR studies of novel and highly active tri-cyclic HIV integrase inhibitors. Bioorg Med Chem Lett16:3989-92 (2006) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Integrase | |||
| Name: | Integrase | ||
| Synonyms: | Human immunodeficiency virus type 1 integrase | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 32231.48 | ||
| Organism: | Human immunodeficiency virus 1 | ||
| Description: | ChEMBL_90865 | ||
| Residue: | 288 | ||
| Sequence: |
| ||
| BDBM50187767 | |||
| n/a | |||
| Name | BDBM50187767 | ||
| Synonyms: | CHEMBL378395 | ethyl 2-(7-(4-fluorobenzyl)-9-hydroxy-8-oxo-7,8-dihydro-6H-pyrrolo[3,4-g]quinolin-5-yl)benzoate | ||
| Type | Small organic molecule | ||
| Emp. Form. | C27H21FN2O4 | ||
| Mol. Mass. | 456.465 | ||
| SMILES | CCOC(=O)c1ccccc1-c1c2CN(Cc3ccc(F)cc3)C(=O)c2c(O)c2ncccc12 |(27.2,-19.28,;28.36,-20.3,;28.07,-21.81,;29.23,-22.82,;28.93,-24.33,;30.69,-22.32,;30.68,-20.78,;32.02,-20,;33.36,-20.77,;33.36,-22.31,;32.02,-23.08,;32.02,-24.62,;30.69,-25.39,;29.22,-24.9,;28.3,-26.16,;26.96,-26.93,;25.63,-26.15,;25.65,-24.61,;24.32,-23.83,;22.98,-24.59,;21.65,-23.81,;22.97,-26.14,;24.3,-26.91,;29.21,-27.4,;28.74,-28.87,;30.69,-26.93,;32.02,-27.7,;32.02,-29.24,;33.34,-26.93,;34.68,-27.7,;36.02,-26.93,;36.01,-25.38,;34.68,-24.63,;33.35,-25.39,)| | ||
| Structure | 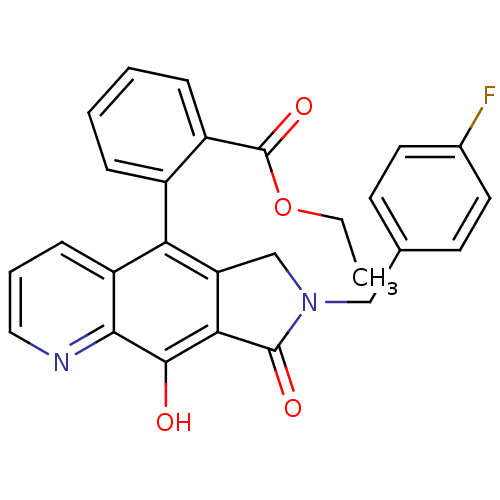
| ||