 Found 66 hits with Last Name = 'fardis' and Initial = 'm'
Found 66 hits with Last Name = 'fardis' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50137405
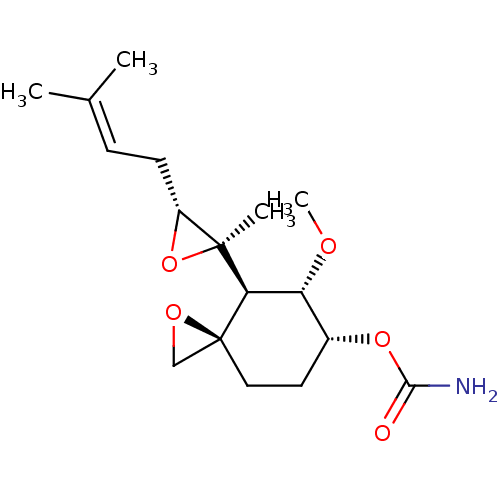
(CHEMBL176975 | Carbamic acid (3R,4S,5S,6R)-5-metho...)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6@H]-1[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](/[#6])-[#6])-[#8]-[#6](-[#7])=O Show InChI InChI=1S/C17H27NO5/c1-10(2)5-6-12-16(3,23-12)14-13(20-4)11(22-15(18)19)7-8-17(14)9-21-17/h5,11-14H,6-9H2,1-4H3,(H2,18,19)/t11-,12-,13-,14-,16+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50137403
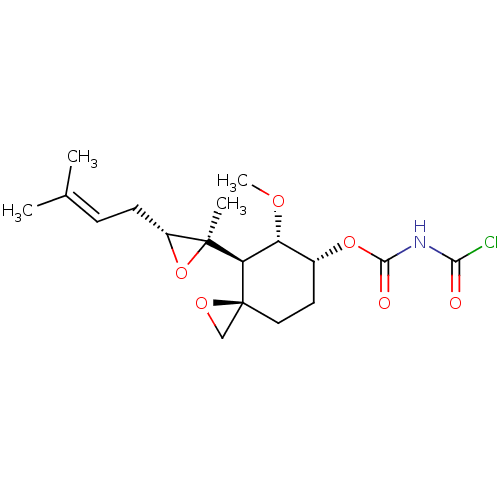
(Acetyl-carbamic acid (3R,4S,5S,6R)-5-methoxy-4-[(R...)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6@H]-1[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](\[#6])-[#6])-[#8]-[#6](=O)-[#7]-[#6](Cl)=O Show InChI InChI=1S/C18H26ClNO6/c1-10(2)5-6-12-17(3,26-12)14-13(23-4)11(7-8-18(14)9-24-18)25-16(22)20-15(19)21/h5,11-14H,6-9H2,1-4H3,(H,20,21,22)/t11-,12-,13-,14-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50137404
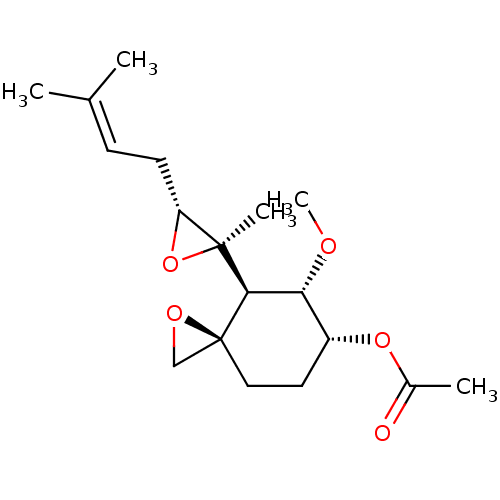
(Acetic acid (3R,4S,5S,6R)-5-methoxy-4-[(2R,3R)-2-m...)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6@H]-1[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](/[#6])-[#6])-[#8]-[#6](-[#6])=O Show InChI InChI=1S/C18H28O5/c1-11(2)6-7-14-17(4,23-14)16-15(20-5)13(22-12(3)19)8-9-18(16)10-21-18/h6,13-16H,7-10H2,1-5H3/t13-,14-,15-,16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50113436
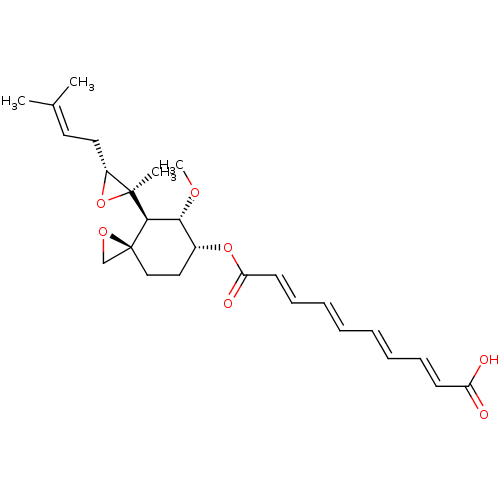
(CHEMBL32838 | fumagillin)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6@H]-1[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](/[#6])-[#6])-[#8]-[#6](=O)\[#6]=[#6]\[#6]=[#6]\[#6]=[#6]\[#6]=[#6]\[#6](-[#8])=O Show InChI InChI=1S/C26H34O7/c1-18(2)13-14-20-25(3,33-20)24-23(30-4)19(15-16-26(24)17-31-26)32-22(29)12-10-8-6-5-7-9-11-21(27)28/h5-13,19-20,23-24H,14-17H2,1-4H3,(H,27,28)/b7-5+,8-6+,11-9+,12-10+/t19-,20-,23-,24-,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50137402

(4-Ethyl-piperazine-1-carboxylic acid (3R,4S,5S,6R)...)Show SMILES CCN1CCN(CC1)C(=O)O[C@@H]1CC[C@]2(CO2)[C@H]([C@@H]1OC)C(\C)=N\OCC1CCCCC1 Show InChI InChI=1S/C24H41N3O5/c1-4-26-12-14-27(15-13-26)23(28)32-20-10-11-24(17-30-24)21(22(20)29-3)18(2)25-31-16-19-8-6-5-7-9-19/h19-22H,4-17H2,1-3H3/b25-18+/t20-,21+,22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50137406
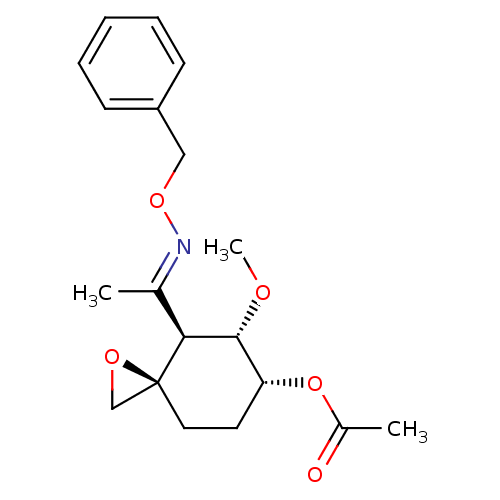
(Acetic acid (3R,4S,5S,6R)-4-{1-[(E)-benzyloxyimino...)Show SMILES CO[C@@H]1[C@@H](CC[C@]2(CO2)[C@H]1\C(C)=N\OCc1ccccc1)OC(C)=O Show InChI InChI=1S/C19H25NO5/c1-13(20-24-11-15-7-5-4-6-8-15)17-18(22-3)16(25-14(2)21)9-10-19(17)12-23-19/h4-8,16-18H,9-12H2,1-3H3/b20-13+/t16-,17+,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50137408

(CHEMBL172883 | Carbamic acid (3R,4S,5S,6R)-4-{1-[(...)Show SMILES CO[C@@H]1[C@@H](CC[C@]2(CO2)[C@H]1\C(C)=N\OCc1ccccc1)OC(N)=O Show InChI InChI=1S/C18H24N2O5/c1-12(20-24-10-13-6-4-3-5-7-13)15-16(22-2)14(25-17(19)21)8-9-18(15)11-23-18/h3-7,14-16H,8-11H2,1-2H3,(H2,19,21)/b20-12+/t14-,15+,16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50137409

(CHEMBL369152 | Carbamic acid (3R,4S,5S,6R)-4-((E)-...)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6@H]-1\[#6](-[#6])=[#6]\[#6]\[#6]=[#6](/[#6])-[#6])-[#8]-[#6](-[#7])=O Show InChI InChI=1S/C17H27NO4/c1-11(2)6-5-7-12(3)14-15(20-4)13(22-16(18)19)8-9-17(14)10-21-17/h6-7,13-15H,5,8-10H2,1-4H3,(H2,18,19)/b12-7+/t13-,14+,15-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187196
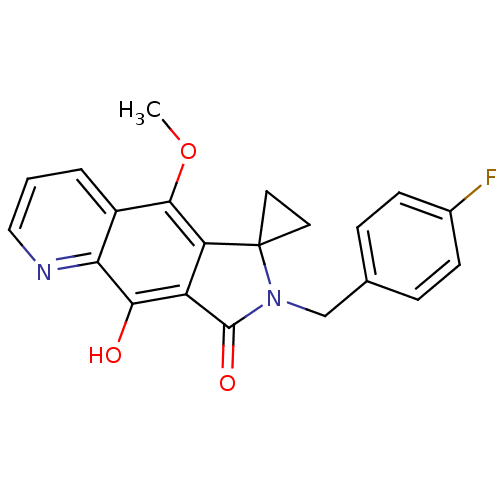
(2'-[(4-fluorophenyl)methyl]-4'-hydroxy-9'-methoxy-...)Show SMILES COc1c2c(C(=O)N(Cc3ccc(F)cc3)C22CC2)c(O)c2ncccc12 Show InChI InChI=1S/C21H17FN2O3/c1-27-19-14-3-2-10-23-17(14)18(25)15-16(19)21(8-9-21)24(20(15)26)11-12-4-6-13(22)7-5-12/h2-7,10,25H,8-9,11H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50137407
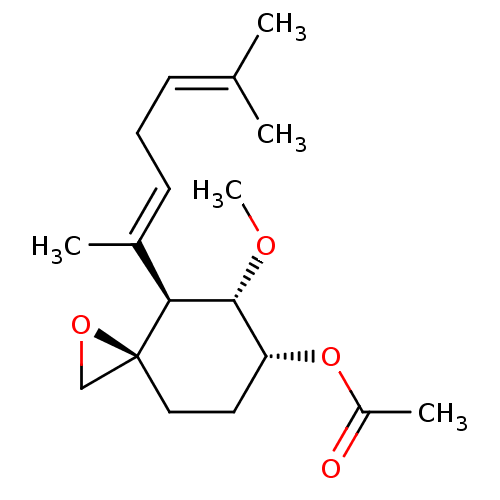
(Acetic acid (3R,4S,5S,6R)-4-((E)-1,5-dimethyl-hexa...)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6@H]-1\[#6](-[#6])=[#6]\[#6]\[#6]=[#6](/[#6])-[#6])-[#8]-[#6](-[#6])=O Show InChI InChI=1S/C18H28O4/c1-12(2)7-6-8-13(3)16-17(20-5)15(22-14(4)19)9-10-18(16)11-21-18/h7-8,15-17H,6,9-11H2,1-5H3/b13-8+/t15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185589
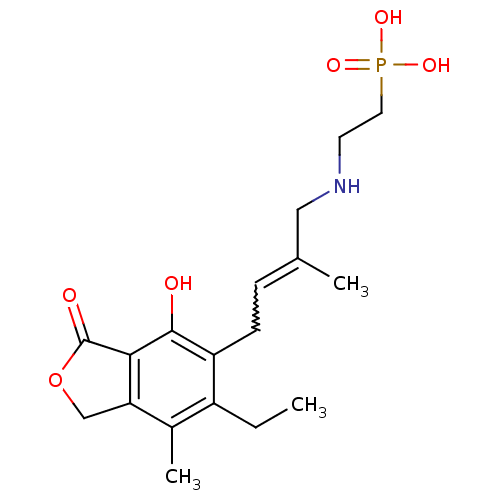
((E)-2-(4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dih...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1CC=C(C)CNCCP(O)(O)=O |w:15.16| Show InChI InChI=1S/C18H26NO6P/c1-4-13-12(3)15-10-25-18(21)16(15)17(20)14(13)6-5-11(2)9-19-7-8-26(22,23)24/h5,19-20H,4,6-10H2,1-3H3,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185586
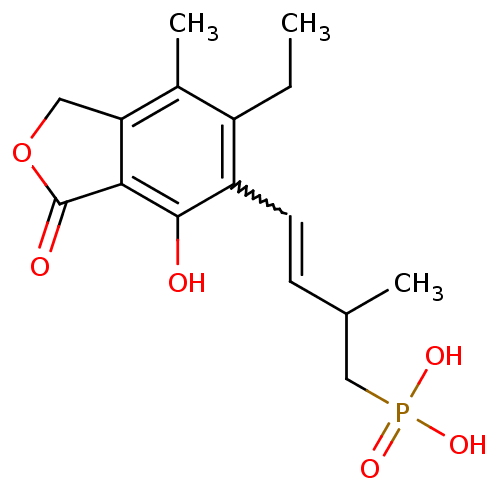
((E)-4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dihydr...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C=CC(C)CP(O)(O)=O |w:14.15| Show InChI InChI=1S/C16H21O6P/c1-4-11-10(3)13-7-22-16(18)14(13)15(17)12(11)6-5-9(2)8-23(19,20)21/h5-6,9,17H,4,7-8H2,1-3H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185592
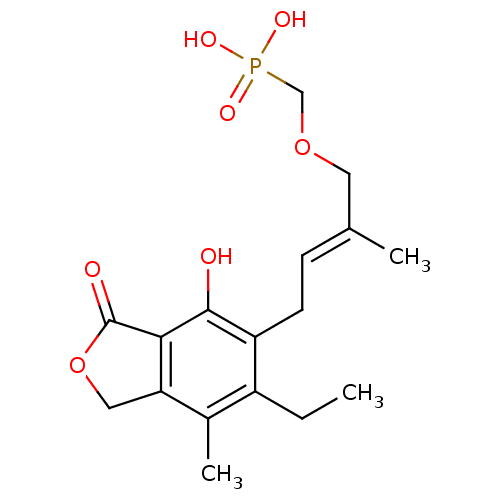
((E)-(4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dihyd...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)COCP(O)(O)=O Show InChI InChI=1S/C17H23O7P/c1-4-12-11(3)14-8-24-17(19)15(14)16(18)13(12)6-5-10(2)7-23-9-25(20,21)22/h5,18H,4,6-9H2,1-3H3,(H2,20,21,22)/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185601
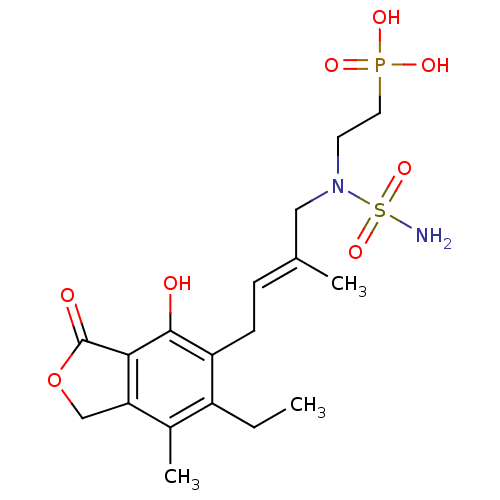
(CHEMBL380059)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(CCP(O)(O)=O)S(N)(=O)=O Show InChI InChI=1S/C18H27N2O8PS/c1-4-13-12(3)15-10-28-18(22)16(15)17(21)14(13)6-5-11(2)9-20(30(19,26)27)7-8-29(23,24)25/h5,21H,4,6-10H2,1-3H3,(H2,19,26,27)(H2,23,24,25)/b11-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50404240

(CHEMBL2110259)Show SMILES CO[C@@H]1[C@@H](CC[C@]2(CO2)[C@H]1\C(C)=N/OCc1ccccc1)OC(C)=O Show InChI InChI=1S/C19H25NO5/c1-13(20-24-11-15-7-5-4-6-8-15)17-18(22-3)16(25-14(2)21)9-10-19(17)12-23-19/h4-8,16-18H,9-12H2,1-3H3/b20-13-/t16-,17+,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187761
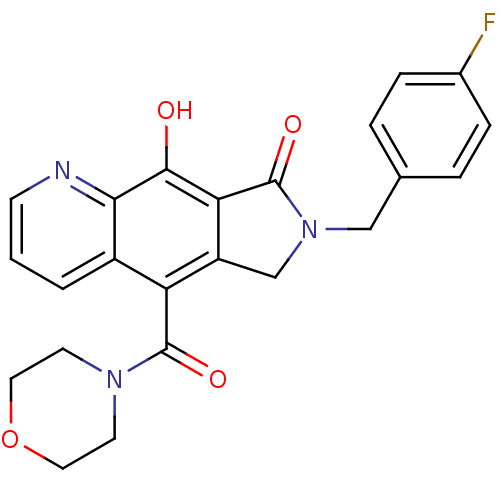
(7-(4-fluorobenzyl)-9-hydroxy-5-(morpholine-4-carbo...)Show SMILES Oc1c2C(=O)N(Cc3ccc(F)cc3)Cc2c(C(=O)N2CCOCC2)c2cccnc12 Show InChI InChI=1S/C23H20FN3O4/c24-15-5-3-14(4-6-15)12-27-13-17-18(22(29)26-8-10-31-11-9-26)16-2-1-7-25-20(16)21(28)19(17)23(27)30/h1-7,28H,8-13H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of strand transfer activity of HIV integrase |
Bioorg Med Chem Lett 16: 3989-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.016
BindingDB Entry DOI: 10.7270/Q29K49T8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187194
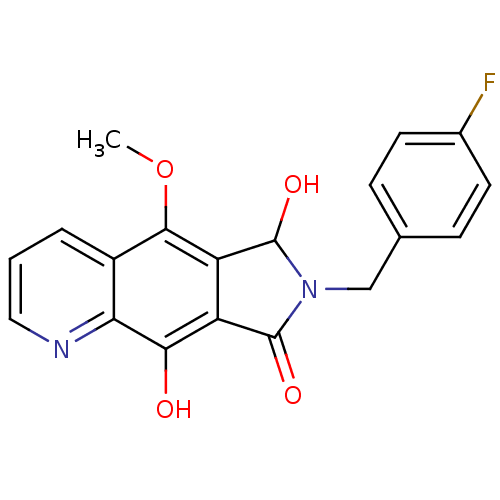
(7-(4-fluorobenzyl)-6,9-dihydroxy-5-methoxy-6,7-dih...)Show SMILES COc1c2C(O)N(Cc3ccc(F)cc3)C(=O)c2c(O)c2ncccc12 Show InChI InChI=1S/C19H15FN2O4/c1-26-17-12-3-2-8-21-15(12)16(23)13-14(17)19(25)22(18(13)24)9-10-4-6-11(20)7-5-10/h2-8,19,23,25H,9H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50185589

((E)-2-(4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dih...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1CC=C(C)CNCCP(O)(O)=O |w:15.16| Show InChI InChI=1S/C18H26NO6P/c1-4-13-12(3)15-10-25-18(21)16(15)17(20)14(13)6-5-11(2)9-19-7-8-26(22,23)24/h5,19-20H,4,6-10H2,1-3H3,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50185592

((E)-(4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dihyd...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)COCP(O)(O)=O Show InChI InChI=1S/C17H23O7P/c1-4-12-11(3)14-8-24-17(19)15(14)16(18)13(12)6-5-10(2)7-23-9-25(20,21)22/h5,18H,4,6-9H2,1-3H3,(H2,20,21,22)/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM23402
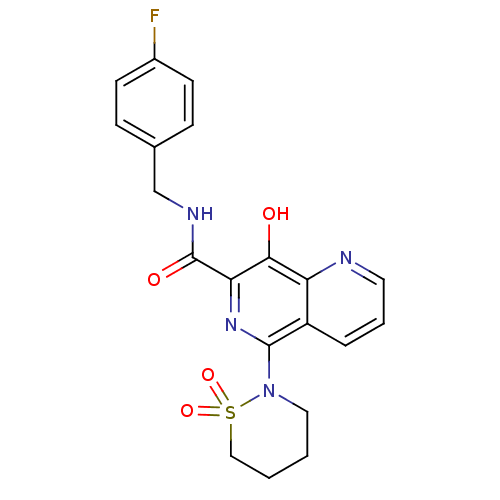
(5-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(4-fluoropheny...)Show SMILES Oc1c(nc(N2CCCCS2(=O)=O)c2cccnc12)C(=O)NCc1ccc(F)cc1 Show InChI InChI=1S/C20H19FN4O4S/c21-14-7-5-13(6-8-14)12-23-20(27)17-18(26)16-15(4-3-9-22-16)19(24-17)25-10-1-2-11-30(25,28)29/h3-9,26H,1-2,10-12H2,(H,23,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of strand transfer activity of HIV integrase |
Bioorg Med Chem Lett 16: 3989-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.016
BindingDB Entry DOI: 10.7270/Q29K49T8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM23403
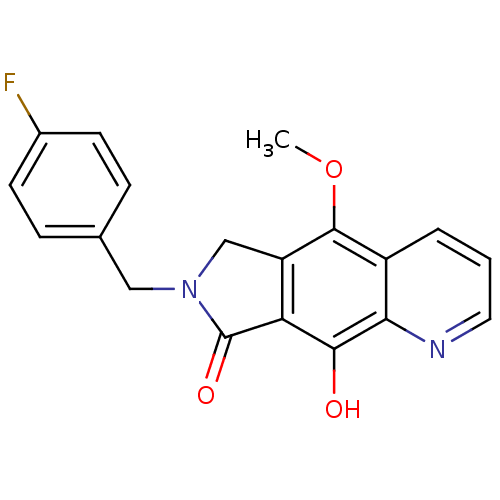
(7-[(4-fluorophenyl)methyl]-9-hydroxy-5-methoxy-6H,...)Show InChI InChI=1S/C19H15FN2O3/c1-25-18-13-3-2-8-21-16(13)17(23)15-14(18)10-22(19(15)24)9-11-4-6-12(20)7-5-11/h2-8,23H,9-10H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of strand transfer activity of HIV integrase |
Bioorg Med Chem Lett 16: 3989-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.016
BindingDB Entry DOI: 10.7270/Q29K49T8 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50404241
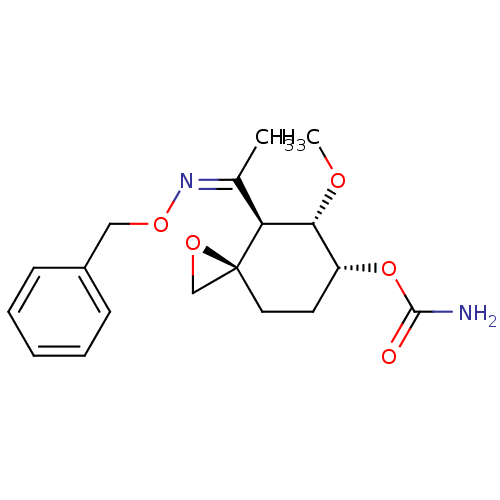
(CHEMBL2110260)Show SMILES CO[C@@H]1[C@@H](CC[C@]2(CO2)[C@H]1\C(C)=N/OCc1ccccc1)OC(N)=O Show InChI InChI=1S/C18H24N2O5/c1-12(20-24-10-13-6-4-3-5-7-13)15-16(22-2)14(25-17(19)21)8-9-18(15)11-23-18/h3-7,14-16H,8-11H2,1-2H3,(H2,19,21)/b20-12-/t14-,15+,16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 2 |
Bioorg Med Chem Lett 14: 91-4 (2003)
BindingDB Entry DOI: 10.7270/Q27P8ZXD |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187198

(7-(4-fluorobenzyl)-9-hydroxy-5-methoxy-6-(3-oxo-3-...)Show SMILES COc1c2C(SCCC(=O)N3CCNCC3)N(Cc3ccc(F)cc3)C(=O)c2c(O)c2ncccc12 Show InChI InChI=1S/C26H27FN4O4S/c1-35-24-18-3-2-9-29-22(18)23(33)20-21(24)26(36-14-8-19(32)30-12-10-28-11-13-30)31(25(20)34)15-16-4-6-17(27)7-5-16/h2-7,9,26,28,33H,8,10-15H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM23403

(7-[(4-fluorophenyl)methyl]-9-hydroxy-5-methoxy-6H,...)Show InChI InChI=1S/C19H15FN2O3/c1-25-18-13-3-2-8-21-16(13)17(23)15-14(18)10-22(19(15)24)9-11-4-6-12(20)7-5-11/h2-8,23H,9-10H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187191

(7-(4-fluorobenzyl)-9-hydroxy-5,6-dimethoxy-6,7-dih...)Show SMILES COC1N(Cc2ccc(F)cc2)C(=O)c2c1c(OC)c1cccnc1c2O Show InChI InChI=1S/C20H17FN2O4/c1-26-18-13-4-3-9-22-16(13)17(24)14-15(18)20(27-2)23(19(14)25)10-11-5-7-12(21)8-6-11/h3-9,20,24H,10H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185595
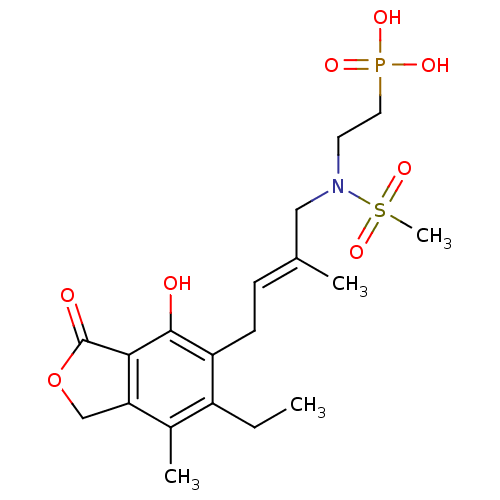
((E)-2-(N-(4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(CCP(O)(O)=O)S(C)(=O)=O Show InChI InChI=1S/C19H28NO8PS/c1-5-14-13(3)16-11-28-19(22)17(16)18(21)15(14)7-6-12(2)10-20(30(4,26)27)8-9-29(23,24)25/h6,21H,5,7-11H2,1-4H3,(H2,23,24,25)/b12-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187760

(7-(4-fluorobenzyl)-9-hydroxy-6,7-dihydropyrrolo[3,...)Show InChI InChI=1S/C18H13FN2O2/c19-14-5-3-11(4-6-14)9-21-10-13-8-12-2-1-7-20-16(12)17(22)15(13)18(21)23/h1-8,22H,9-10H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of strand transfer activity of HIV integrase |
Bioorg Med Chem Lett 16: 3989-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.016
BindingDB Entry DOI: 10.7270/Q29K49T8 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50185586

((E)-4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dihydr...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C=CC(C)CP(O)(O)=O |w:14.15| Show InChI InChI=1S/C16H21O6P/c1-4-11-10(3)13-7-22-16(18)14(13)15(17)12(11)6-5-9(2)8-23(19,20)21/h5-6,9,17H,4,7-8H2,1-3H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187764

(7-(4-fluorobenzyl)-9-hydroxy-5-methoxy-6H-pyrrolo[...)Show SMILES COc1c2C(=O)N(Cc3ccc(F)cc3)C(=O)c2c(O)c2ncccc12 Show InChI InChI=1S/C19H13FN2O4/c1-26-17-12-3-2-8-21-15(12)16(23)13-14(17)19(25)22(18(13)24)9-10-4-6-11(20)7-5-10/h2-8,23H,9H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of strand transfer activity of HIV integrase |
Bioorg Med Chem Lett 16: 3989-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.016
BindingDB Entry DOI: 10.7270/Q29K49T8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187186

(7-(4-fluorobenzyl)-5,9-dihydroxy-7H-pyrrolo[3,4-g]...)Show SMILES Oc1c2C(=O)N(Cc3ccc(F)cc3)C(=O)c2c(O)c2ncccc12 Show InChI InChI=1S/C18H11FN2O4/c19-10-5-3-9(4-6-10)8-21-17(24)12-13(18(21)25)16(23)14-11(15(12)22)2-1-7-20-14/h1-7,22-23H,8H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185585

((E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihy...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CC=C(C)CCCP(O)(O)=O |w:15.16| Show InChI InChI=1S/C17H23O7P/c1-10(5-4-8-25(20,21)22)6-7-12-15(18)14-13(9-24-17(14)19)11(2)16(12)23-3/h6,18H,4-5,7-9H2,1-3H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187190
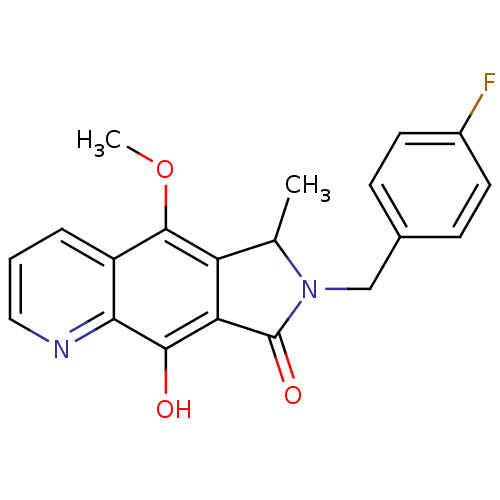
(7-(4-fluorobenzyl)-9-hydroxy-5-methoxy-6-methyl-6,...)Show SMILES COc1c2C(C)N(Cc3ccc(F)cc3)C(=O)c2c(O)c2ncccc12 Show InChI InChI=1S/C20H17FN2O3/c1-11-15-16(18(24)17-14(19(15)26-2)4-3-9-22-17)20(25)23(11)10-12-5-7-13(21)8-6-12/h3-9,11,24H,10H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185590

((2-{[(2E)-4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(CCP(O)(O)=O)S(N)(=O)=O Show InChI InChI=1S/C17H25N2O9PS/c1-10(8-19(30(18,25)26)6-7-29(22,23)24)4-5-12-15(20)14-13(9-28-17(14)21)11(2)16(12)27-3/h4,20H,5-9H2,1-3H3,(H2,18,25,26)(H2,22,23,24)/b10-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185596
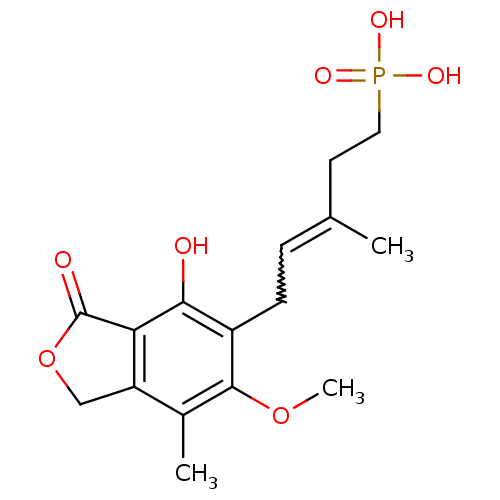
((E)-5-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihy...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1CC=C(C)CCP(O)(O)=O |w:15.16| Show InChI InChI=1S/C16H21O7P/c1-9(6-7-24(19,20)21)4-5-11-14(17)13-12(8-23-16(13)18)10(2)15(11)22-3/h4,17H,5-8H2,1-3H3,(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185604

((E)-2-((4-(6-ethyl-4-hydroxy-7-methyl-3-oxo-1,3-di...)Show SMILES CCc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CN(C)CCP(O)(O)=O Show InChI InChI=1S/C19H28NO6P/c1-5-14-13(3)16-11-26-19(22)17(16)18(21)15(14)7-6-12(2)10-20(4)8-9-27(23,24)25/h6,21H,5,7-11H2,1-4H3,(H2,23,24,25)/b12-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187185

(7-(4-fluorobenzyl)-9-hydroxy-5-methoxy-6-methylene...)Show SMILES COc1c2C(=C)N(Cc3ccc(F)cc3)C(=O)c2c(O)c2ncccc12 Show InChI InChI=1S/C20H15FN2O3/c1-11-15-16(18(24)17-14(19(15)26-2)4-3-9-22-17)20(25)23(11)10-12-5-7-13(21)8-6-12/h3-9,24H,1,10H2,2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185603

((E)-4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihy...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C=CC(C)CP(O)(O)=O |w:14.15| Show InChI InChI=1S/C15H19O7P/c1-8(7-23(18,19)20)4-5-10-13(16)12-11(6-22-15(12)17)9(2)14(10)21-3/h4-5,8,16H,6-7H2,1-3H3,(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187759
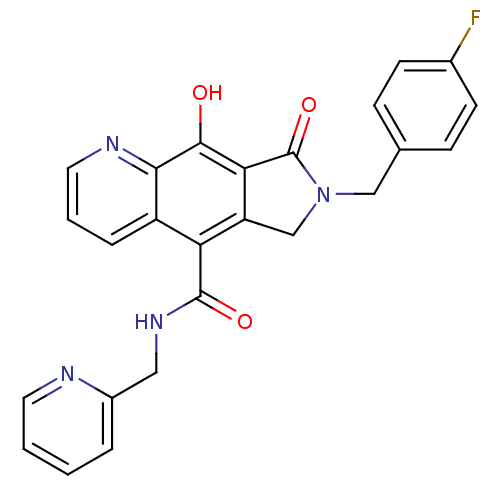
(7-(4-fluorobenzyl)-9-hydroxy-8-oxo-N-(pyridin-2-yl...)Show SMILES Oc1c2C(=O)N(Cc3ccc(F)cc3)Cc2c(C(=O)NCc2ccccn2)c2cccnc12 Show InChI InChI=1S/C25H19FN4O3/c26-16-8-6-15(7-9-16)13-30-14-19-20(24(32)29-12-17-4-1-2-10-27-17)18-5-3-11-28-22(18)23(31)21(19)25(30)33/h1-11,31H,12-14H2,(H,29,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of strand transfer activity of HIV integrase |
Bioorg Med Chem Lett 16: 3989-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.016
BindingDB Entry DOI: 10.7270/Q29K49T8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187193
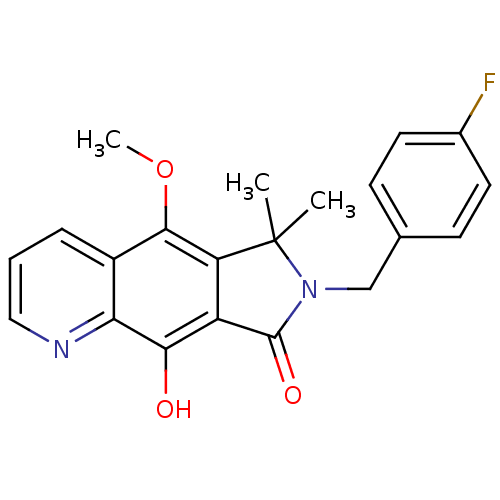
(7-(4-fluorobenzyl)-9-hydroxy-5-methoxy-6,6-dimethy...)Show SMILES COc1c2c(C(=O)N(Cc3ccc(F)cc3)C2(C)C)c(O)c2ncccc12 Show InChI InChI=1S/C21H19FN2O3/c1-21(2)16-15(18(25)17-14(19(16)27-3)5-4-10-23-17)20(26)24(21)11-12-6-8-13(22)9-7-12/h4-10,25H,11H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185599
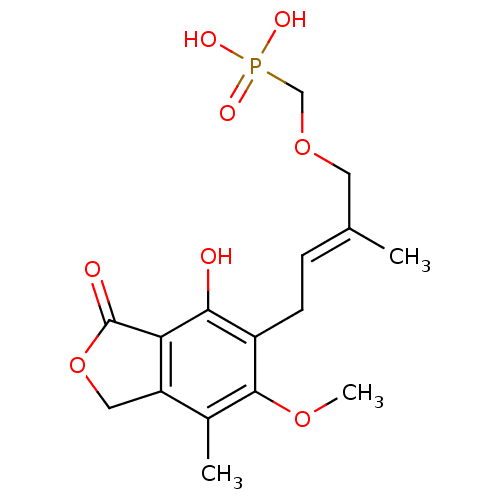
((E)-(4-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)COCP(O)(O)=O Show InChI InChI=1S/C16H21O8P/c1-9(6-23-8-25(19,20)21)4-5-11-14(17)13-12(7-24-16(13)18)10(2)15(11)22-3/h4,17H,5-8H2,1-3H3,(H2,19,20,21)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185587

(7-hydroxy-5-methoxy-4-methyl-6-(3-methylbut-2-enyl...)Show SMILES [#6]-[#8]-c1c(-[#6])c2-[#6]-[#8]-[#6](=O)-c2c(-[#8])c1-[#6]\[#6]=[#6](/[#6])-[#6] Show InChI InChI=1S/C15H18O4/c1-8(2)5-6-10-13(16)12-11(7-19-15(12)17)9(3)14(10)18-4/h5,16H,6-7H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187187
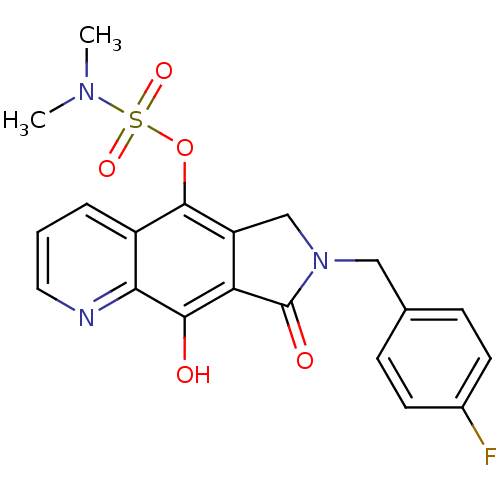
(7-(4-fluorobenzyl)-9-hydroxy-8-oxo-7,8-dihydro-6H-...)Show SMILES CN(C)S(=O)(=O)Oc1c2CN(Cc3ccc(F)cc3)C(=O)c2c(O)c2ncccc12 Show InChI InChI=1S/C20H18FN3O5S/c1-23(2)30(27,28)29-19-14-4-3-9-22-17(14)18(25)16-15(19)11-24(20(16)26)10-12-5-7-13(21)8-6-12/h3-9,25H,10-11H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185594

((E)-7-hydroxy-5-methoxy-6-(4-methoxy-3-methylbut-2...)Show InChI InChI=1S/C16H20O5/c1-9(7-19-3)5-6-11-14(17)13-12(8-21-16(13)18)10(2)15(11)20-4/h5,17H,6-8H2,1-4H3/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50185588
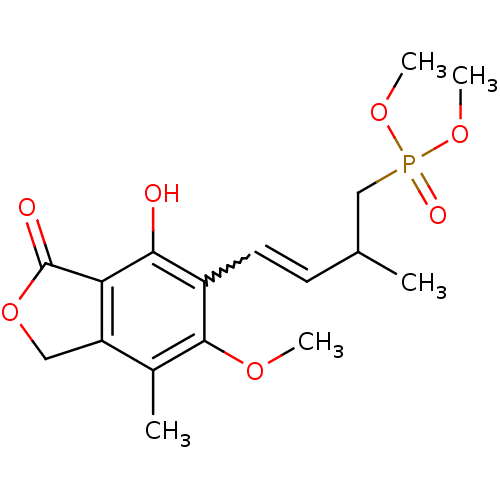
((E)-dimethyl 4-(4-hydroxy-6-methoxy-7-methyl-3-oxo...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C=CC(C)CP(=O)(OC)OC |w:14.15| Show InChI InChI=1S/C17H23O7P/c1-10(9-25(20,22-4)23-5)6-7-12-15(18)14-13(8-24-17(14)19)11(2)16(12)21-3/h6-7,10,18H,8-9H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 289 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 |
Bioorg Med Chem Lett 16: 3479-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.097
BindingDB Entry DOI: 10.7270/Q2HH6JP9 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187197
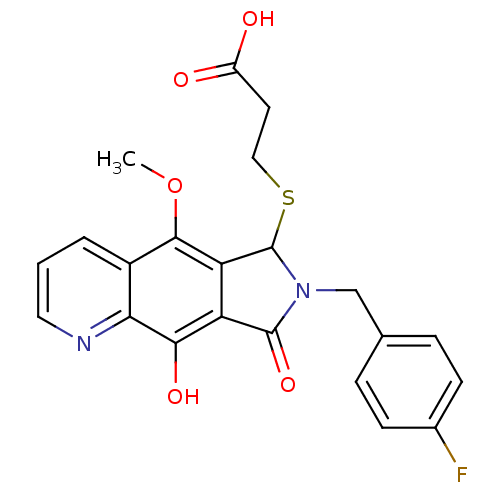
(3-(7-(4-fluorobenzyl)-9-hydroxy-5-methoxy-8-oxo-7,...)Show SMILES COc1c2C(SCCC(O)=O)N(Cc3ccc(F)cc3)C(=O)c2c(O)c2ncccc12 Show InChI InChI=1S/C22H19FN2O5S/c1-30-20-14-3-2-9-24-18(14)19(28)16-17(20)22(31-10-8-15(26)27)25(21(16)29)11-12-4-6-13(23)7-5-12/h2-7,9,22,28H,8,10-11H2,1H3,(H,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187188
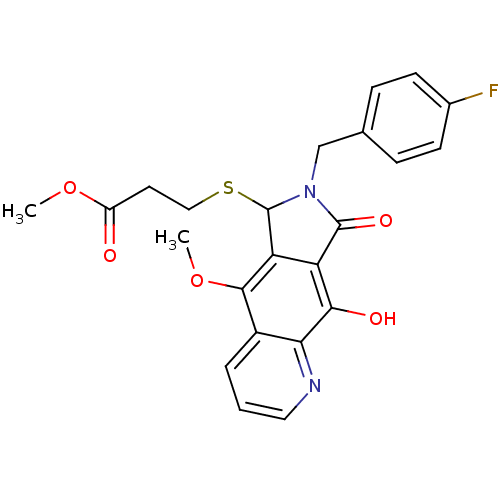
(CHEMBL209641 | methyl 3-(7-(4-fluorobenzyl)-9-hydr...)Show SMILES COC(=O)CCSC1N(Cc2ccc(F)cc2)C(=O)c2c1c(OC)c1cccnc1c2O Show InChI InChI=1S/C23H21FN2O5S/c1-30-16(27)9-11-32-23-18-17(20(28)19-15(21(18)31-2)4-3-10-25-19)22(29)26(23)12-13-5-7-14(24)8-6-13/h3-8,10,23,28H,9,11-12H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer activity in MT4 cell line |
Bioorg Med Chem Lett 16: 4031-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.008
BindingDB Entry DOI: 10.7270/Q2QR4WRV |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187765

(5-(2,6-difluorophenyl)-7-(4-fluorobenzyl)-9-hydrox...)Show SMILES Oc1c2C(=O)N(Cc3ccc(F)cc3)Cc2c(-c2c(F)cccc2F)c2cccnc12 |(-1.61,-44.23,;-1.62,-42.69,;-2.95,-41.93,;-4.42,-42.4,;-4.9,-43.87,;-5.33,-41.15,;-6.67,-41.92,;-8,-41.14,;-7.98,-39.61,;-9.31,-38.83,;-10.65,-39.59,;-11.98,-38.81,;-10.66,-41.14,;-9.33,-41.91,;-4.41,-39.9,;-2.94,-40.38,;-1.61,-39.61,;-1.61,-38.07,;-.28,-37.31,;1.06,-38.08,;-.28,-35.77,;-1.61,-35,;-2.95,-35.78,;-2.94,-37.32,;-4.27,-38.09,;-.28,-40.39,;1.04,-39.61,;2.38,-40.37,;2.39,-41.93,;1.04,-42.7,;-.29,-41.93,)| Show InChI InChI=1S/C24H15F3N2O2/c25-14-8-6-13(7-9-14)11-29-12-16-19(21-17(26)4-1-5-18(21)27)15-3-2-10-28-22(15)23(30)20(16)24(29)31/h1-10,30H,11-12H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 335 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of strand transfer activity of HIV integrase |
Bioorg Med Chem Lett 16: 3989-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.016
BindingDB Entry DOI: 10.7270/Q29K49T8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50187767
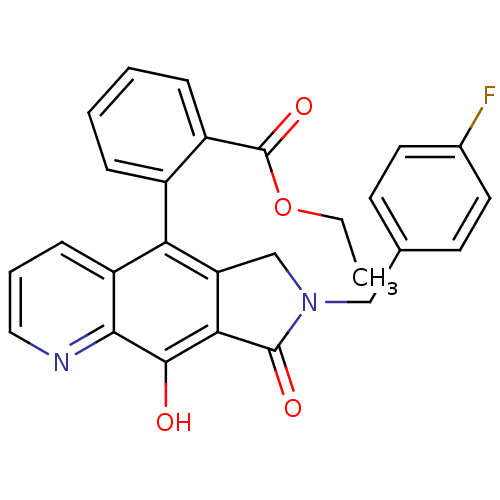
(CHEMBL378395 | ethyl 2-(7-(4-fluorobenzyl)-9-hydro...)Show SMILES CCOC(=O)c1ccccc1-c1c2CN(Cc3ccc(F)cc3)C(=O)c2c(O)c2ncccc12 |(27.2,-19.28,;28.36,-20.3,;28.07,-21.81,;29.23,-22.82,;28.93,-24.33,;30.69,-22.32,;30.68,-20.78,;32.02,-20,;33.36,-20.77,;33.36,-22.31,;32.02,-23.08,;32.02,-24.62,;30.69,-25.39,;29.22,-24.9,;28.3,-26.16,;26.96,-26.93,;25.63,-26.15,;25.65,-24.61,;24.32,-23.83,;22.98,-24.59,;21.65,-23.81,;22.97,-26.14,;24.3,-26.91,;29.21,-27.4,;28.74,-28.87,;30.69,-26.93,;32.02,-27.7,;32.02,-29.24,;33.34,-26.93,;34.68,-27.7,;36.02,-26.93,;36.01,-25.38,;34.68,-24.63,;33.35,-25.39,)| Show InChI InChI=1S/C27H21FN2O4/c1-2-34-27(33)19-7-4-3-6-18(19)22-20-8-5-13-29-24(20)25(31)23-21(22)15-30(26(23)32)14-16-9-11-17(28)12-10-16/h3-13,31H,2,14-15H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of strand transfer activity of HIV integrase |
Bioorg Med Chem Lett 16: 3989-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.016
BindingDB Entry DOI: 10.7270/Q29K49T8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data