| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glutamate receptor ionotropic, NMDA 2B |
|---|
| Ligand | BDBM50220576 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_457335 (CHEMBL940926) |
|---|
| IC50 | 11±n/a nM |
|---|
| Citation |  Kawai, M; Ando, K; Matsumoto, Y; Sakurada, I; Hirota, M; Nakamura, H; Ohta, A; Sudo, M; Hattori, K; Takashima, T; Hizue, M; Watanabe, S; Fujita, I; Mizutani, M; Kawamura, M Discovery of (-)-6-[2-[4-(3-fluorophenyl)-4-hydroxy-1-piperidinyl]-1-hydroxyethyl]-3,4-dihydro-2(1H)-quinolinone--a potent NR2B-selective N-methyl D-aspartate (NMDA) antagonist for the treatment of pain. Bioorg Med Chem Lett17:5558-62 (2007) [PubMed] Article Kawai, M; Ando, K; Matsumoto, Y; Sakurada, I; Hirota, M; Nakamura, H; Ohta, A; Sudo, M; Hattori, K; Takashima, T; Hizue, M; Watanabe, S; Fujita, I; Mizutani, M; Kawamura, M Discovery of (-)-6-[2-[4-(3-fluorophenyl)-4-hydroxy-1-piperidinyl]-1-hydroxyethyl]-3,4-dihydro-2(1H)-quinolinone--a potent NR2B-selective N-methyl D-aspartate (NMDA) antagonist for the treatment of pain. Bioorg Med Chem Lett17:5558-62 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glutamate receptor ionotropic, NMDA 2B |
|---|
| Name: | Glutamate receptor ionotropic, NMDA 2B |
|---|
| Synonyms: | GluN2B | Glutamate [NMDA] receptor subunit epsilon 2 | Grin2b | N-methyl D-aspartate receptor subtype 2B | NMDA receptor subunit N2B (GluN2B) | NMDAR2B | NMDE2_RAT | NR2B |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 166077.66 |
|---|
| Organism: | Rattus norvegicus (Rat) |
|---|
| Description: | Q00960 |
|---|
| Residue: | 1482 |
|---|
| Sequence: | MKPSAECCSPKFWLVLAVLAVSGSKARSQKSPPSIGIAVILVGTSDEVAIKDAHEKDDFH
HLSVVPRVELVAMNETDPKSIITRICDLMSDRKIQGVVFADDTDQEAIAQILDFISAQTL
TPILGIHGGSSMIMADKDESSMFFQFGPSIEQQASVMLNIMEEYDWYIFSIVTTYFPGYQ
DFVNKIRSTIENSFVGWELEEVLLLDMSLDDGDSKIQNQLKKLQSPIILLYCTKEEATYI
FEVANSVGLTGYGYTWIVPSLVAGDTDTVPSEFPTGLISVSYDEWDYGLPARVRDGIAII
TTAASDMLSEHSFIPEPKSSCYNTHEKRIYQSNMLNRYLINVTFEGRNLSFSEDGYQMHP
KLVIILLNKERKWERVGKWKDKSLQMKYYVWPRMCPETEEQEDDHLSIVTLEEAPFVIVE
SVDPLSGTCMRNTVPCQKRIISENKTDEEPGYIKKCCKGFCIDILKKISKSVKFTYDLYL
VTNGKHGKKINGTWNGMIGEVVMKRAYMAVGSLTINEERSEVVDFSVPFIETGISVMVSR
SNGTVSPSAFLEPFSADVWVMMFVMLLIVSAVAVFVFEYFSPVGYNRCLADGREPGGPSF
TIGKAIWLLWGLVFNNSVPVQNPKGTTSKIMVSVWAFFAVIFLASYTANLAAFMIQEEYV
DQVSGLSDKKFQRPNDFSPPFRFGTVPNGSTERNIRNNYAEMHAYMGKFNQRGVDDALLS
LKTGKLDAFIYDAAVLNYMAGRDEGCKLVTIGSGKVFASTGYGIAIQKDSGWKRQVDLAI
LQLFGDGEMEELEALWLTGICHNEKNEVMSSQLDIDNMAGVFYMLGAAMALSLITFICEH
LFYWQFRHCFMGVCSGKPGMVFSISRGIYSCIHGVAIEERQSVMNSPTATMNNTHSNILR
LLRTAKNMANLSGVNGSPQSALDFIRRESSVYDISEHRRSFTHSDCKSYNNPPCEENLFS
DYISEVERTFGNLQLKDSNVYQDHYHHHHRPHSIGSTSSIDGLYDCDNPPFTTQPRSISK
KPLDIGLPSSKHSQLSDLYGKFSFKSDRYSGHDDLIRSDVSDISTHTVTYGNIEGNAAKR
RKQQYKDSLKKRPASAKSRREFDEIELAYRRRPPRSPDHKRYFRDKEGLRDFYLDQFRTK
ENSPHWEHVDLTDIYKERSDDFKRDSVSGGGPCTNRSHLKHGTGEKHGVVGGVPAPWEKN
LTNVDWEDRSGGNFCRSCPSKLHNYSSTVAGQNSGRQACIRCEACKKAGNLYDISEDNSL
QELDQPAAPVAVTSNASSTKYPQSPTNSKAQKKNRNKLRRQHSYDTFVDLQKEEAALAPR
SVSLKDKGRFMDGSPYAHMFEMPAGESSFANKSSVPTAGHHHNNPGSGYMLSKSLYPDRV
TQNPFIPTFGDDQCLLHGSKSYFFRQPTVAGASKTRPDFRALVTNKPVVVTLHGAVPGRF
QKDICIGNQSNPCVPNNKNPRAFNGSSNGHVYEKLSSIESDV
|
|
|
|---|
| BDBM50220576 |
|---|
| n/a |
|---|
| Name | BDBM50220576 |
|---|
| Synonyms: | 6-(2-(4-(4-fluorophenyl)-4-hydroxypiperidin-1-yl)-1-hydroxypropyl)-3,4-dihydroquinolin-2(1H)-one | CHEMBL404851 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H27FN2O3 |
|---|
| Mol. Mass. | 398.4705 |
|---|
| SMILES | CC(C(O)c1ccc2NC(=O)CCc2c1)N1CCC(O)(CC1)c1ccc(F)cc1 |w:2.2,1.0| |
|---|
| Structure | 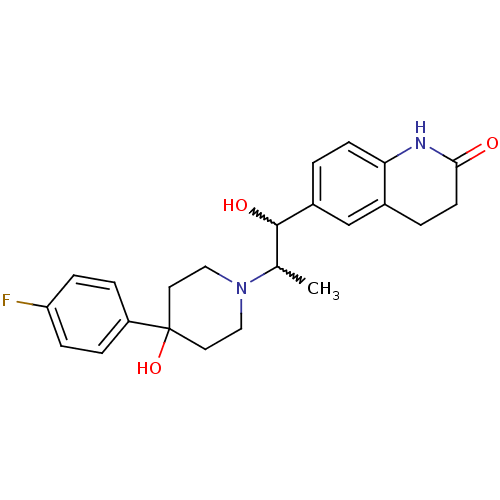
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kawai, M; Ando, K; Matsumoto, Y; Sakurada, I; Hirota, M; Nakamura, H; Ohta, A; Sudo, M; Hattori, K; Takashima, T; Hizue, M; Watanabe, S; Fujita, I; Mizutani, M; Kawamura, M Discovery of (-)-6-[2-[4-(3-fluorophenyl)-4-hydroxy-1-piperidinyl]-1-hydroxyethyl]-3,4-dihydro-2(1H)-quinolinone--a potent NR2B-selective N-methyl D-aspartate (NMDA) antagonist for the treatment of pain. Bioorg Med Chem Lett17:5558-62 (2007) [PubMed] Article
Kawai, M; Ando, K; Matsumoto, Y; Sakurada, I; Hirota, M; Nakamura, H; Ohta, A; Sudo, M; Hattori, K; Takashima, T; Hizue, M; Watanabe, S; Fujita, I; Mizutani, M; Kawamura, M Discovery of (-)-6-[2-[4-(3-fluorophenyl)-4-hydroxy-1-piperidinyl]-1-hydroxyethyl]-3,4-dihydro-2(1H)-quinolinone--a potent NR2B-selective N-methyl D-aspartate (NMDA) antagonist for the treatment of pain. Bioorg Med Chem Lett17:5558-62 (2007) [PubMed] Article