

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237869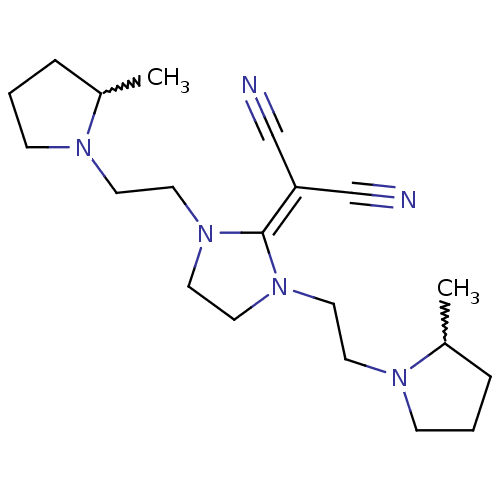 ((+/-)-2-(1,3-bis(2-(2-methylpyrrolidin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237870 (2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237874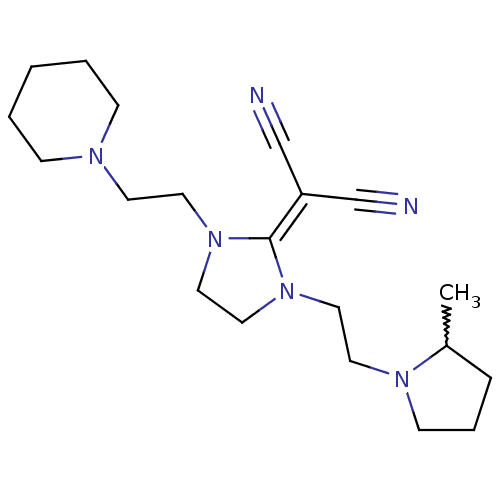 ((+/-)-2-(1-(2-(2-methylpyrrolidin-1-yl)ethyl)-3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237872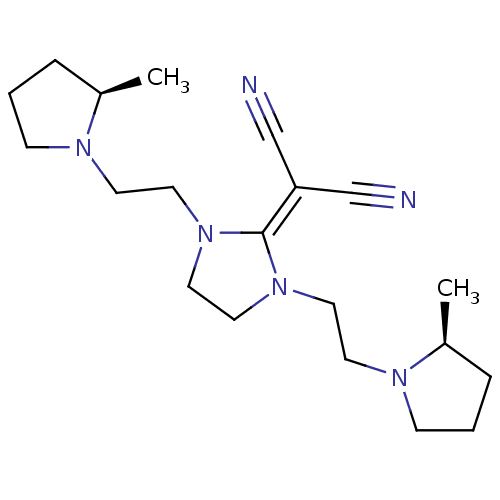 (2-(3-(2-((R)-2-methylpyrrolidin-1-yl)ethyl)-1-(2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237870 (2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237869 ((+/-)-2-(1,3-bis(2-(2-methylpyrrolidin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237874 ((+/-)-2-(1-(2-(2-methylpyrrolidin-1-yl)ethyl)-3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237872 (2-(3-(2-((R)-2-methylpyrrolidin-1-yl)ethyl)-1-(2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237875 (2-(1,3-bis(2-((R)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237875 (2-(1,3-bis(2-((R)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237878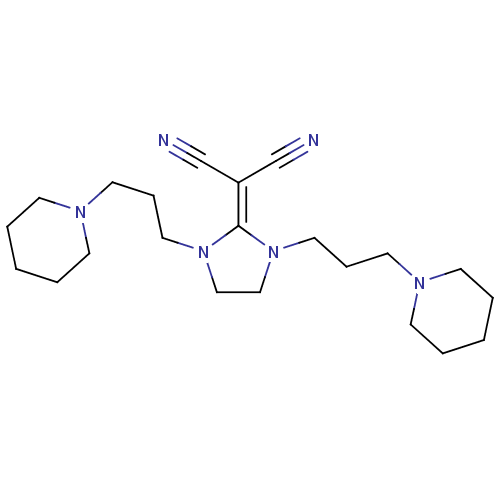 (2-(1,3-bis(3-(piperidin-1-yl)propyl)imidazolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237878 (2-(1,3-bis(3-(piperidin-1-yl)propyl)imidazolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220612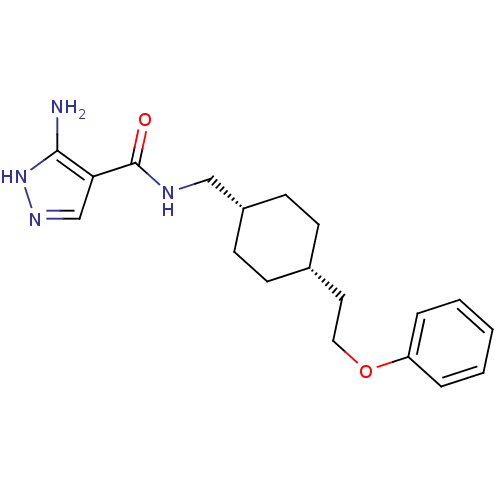 (3-amino-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220606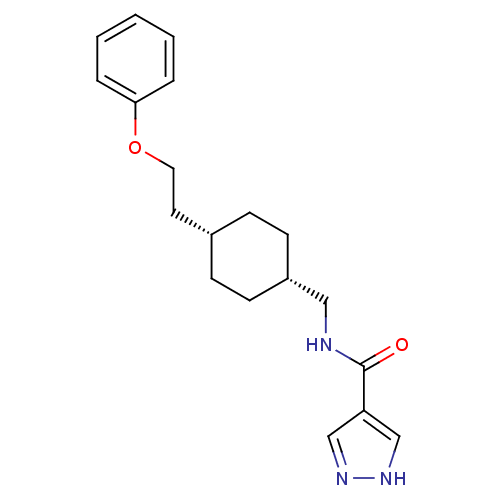 (CHEMBL249093 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220592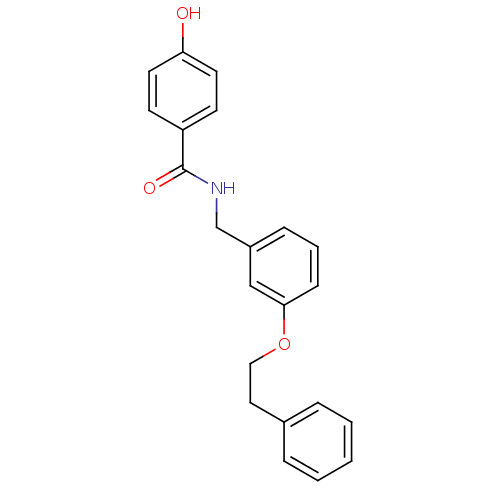 (CHEMBL248875 | N-(3-phenethoxybenzyl)-4-hydroxyben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220611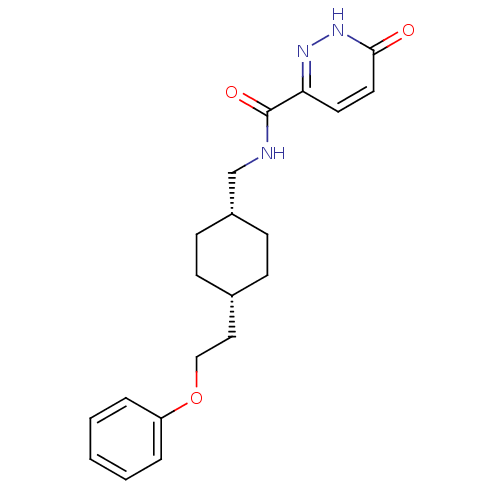 (6-hydroxy-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220608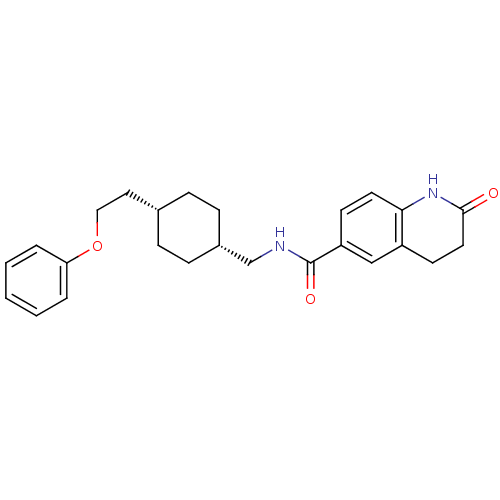 (2-oxo-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237871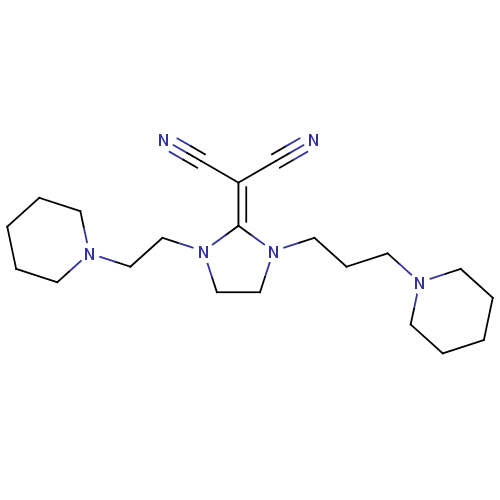 (2-(1-(2-(piperidin-1-yl)ethyl)-3-(3-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237871 (2-(1-(2-(piperidin-1-yl)ethyl)-3-(3-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220603 (4-hydroxy-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220602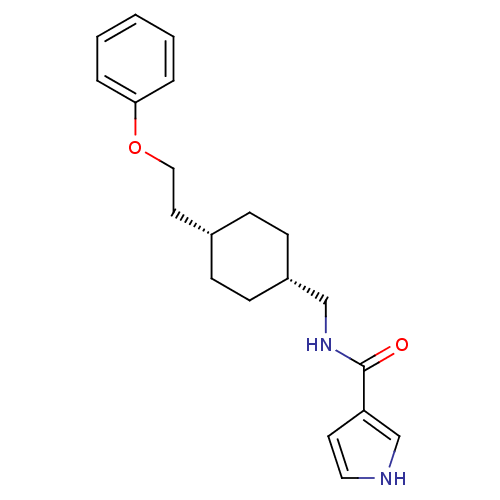 (CHEMBL249307 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50231646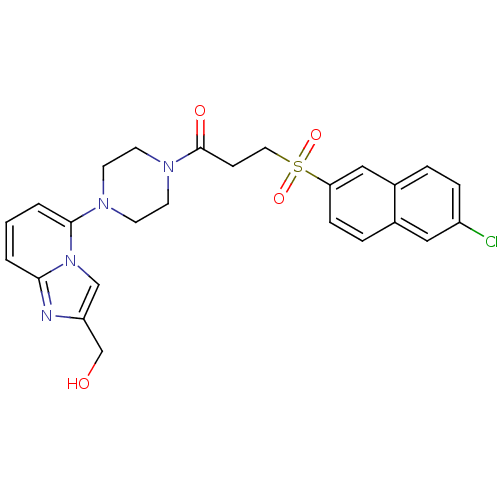 (3-(6-Chloro-naphthalene-2-sulfonyl)-1-[4-(2-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem 16: 3125-40 (2008) Article DOI: 10.1016/j.bmc.2007.12.024 BindingDB Entry DOI: 10.7270/Q2VQ33J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220595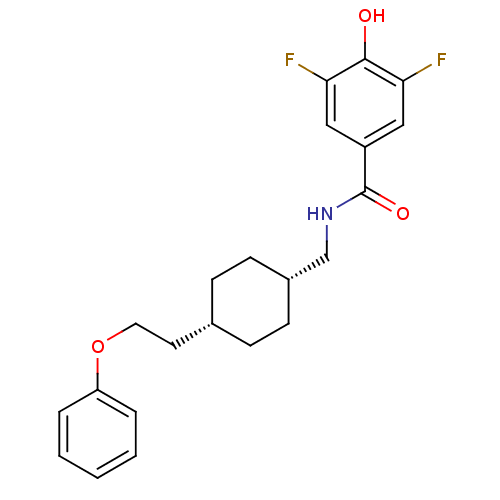 (3,5-difluoro-4-hydroxy-N-(((1s,4s)-4-(2-phenoxyeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237873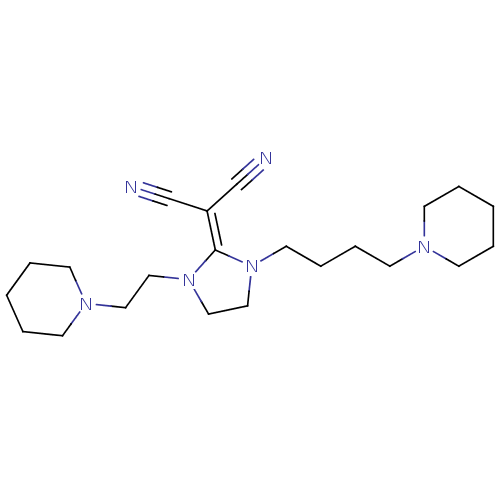 (2-(1-(4-(piperidin-1-yl)butyl)-3-(2-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220591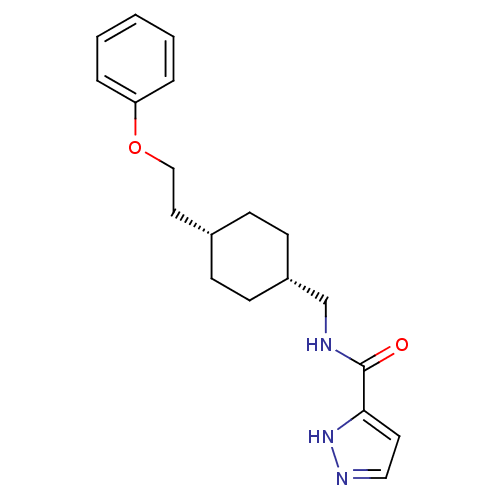 (CHEMBL249909 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220598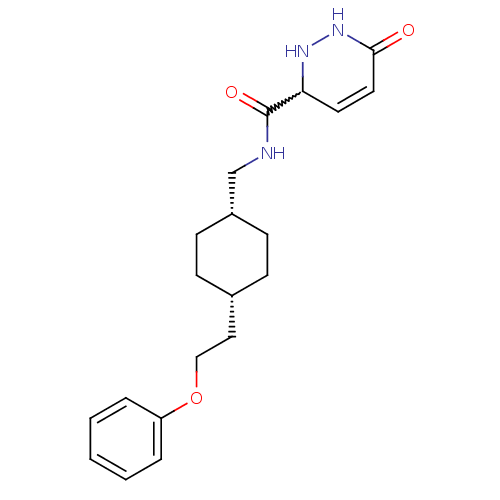 (6-oxo-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220599 (3,5-difluoro-4-hydroxy-N-(((1s,4s)-4-(phenoxymethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220610 (CHEMBL250108 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220601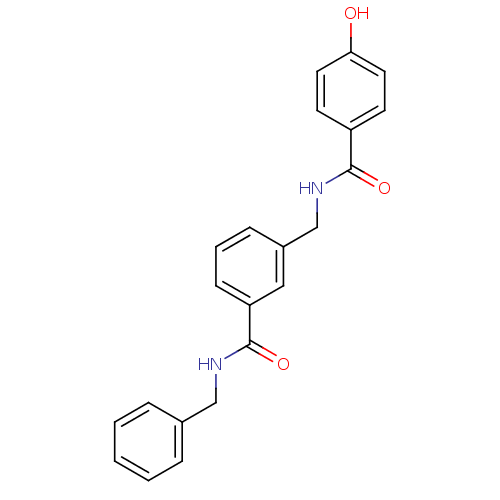 (CHEMBL400917 | N-benzyl-3-{[(4-hydroxybenzoyl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220597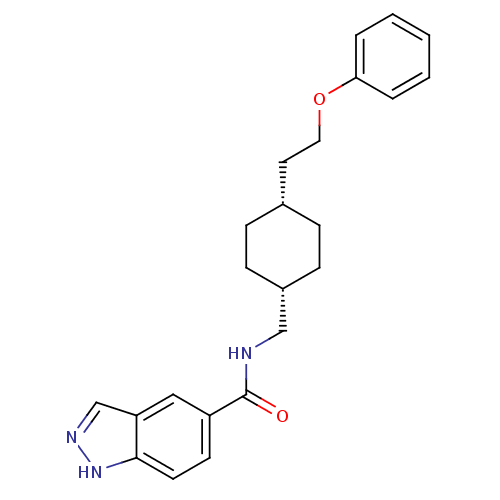 (CHEMBL398356 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50372526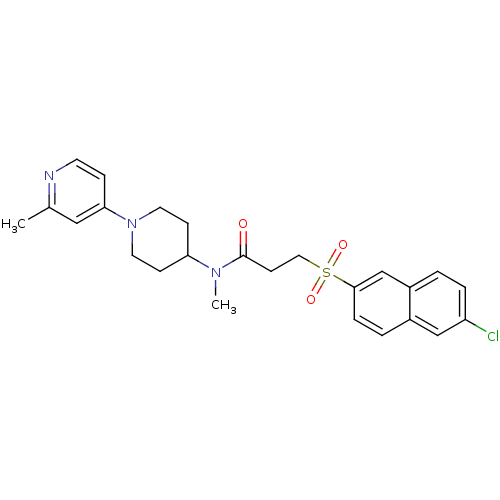 (CHEMBL261646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem 16: 2243-60 (2008) Article DOI: 10.1016/j.bmc.2007.11.073 BindingDB Entry DOI: 10.7270/Q2ZC83QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237873 (2-(1-(4-(piperidin-1-yl)butyl)-3-(2-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220607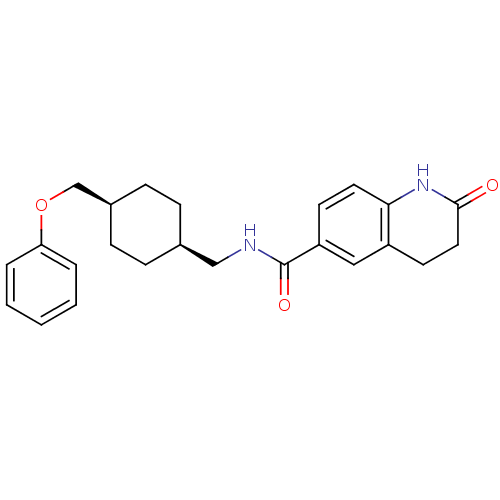 (2-oxo-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220590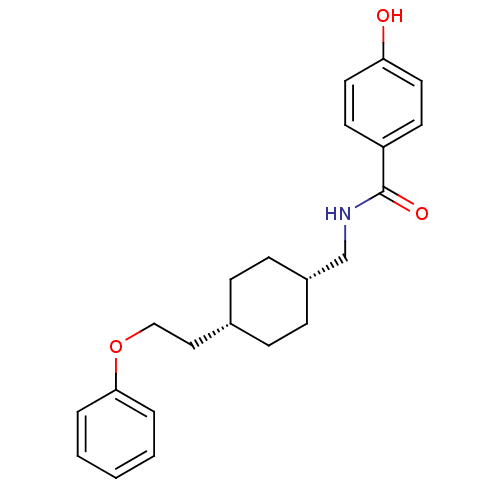 (4-hydroxy-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237879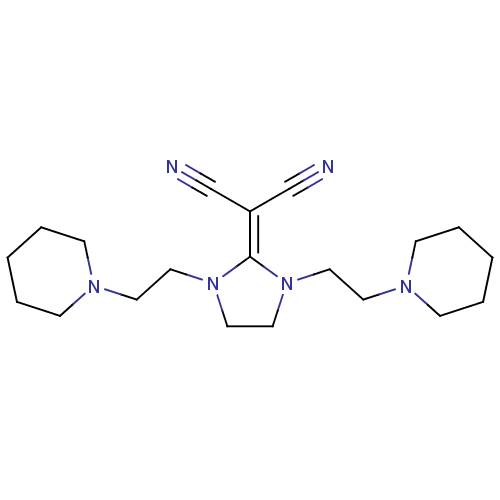 (2-(1,3-bis(2-(piperidin-1-yl)ethyl)imidazolidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237876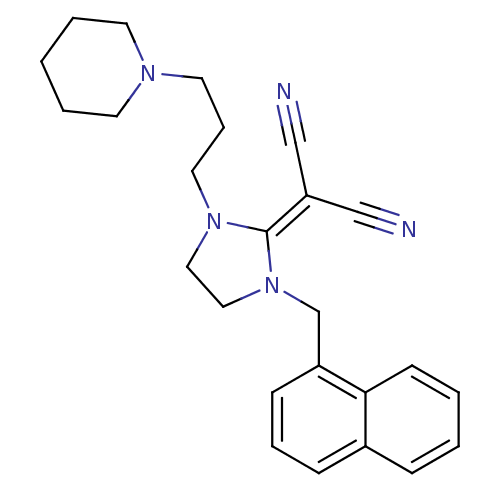 (2-(1-(naphthalen-1-ylmethyl)-3-(3-(piperidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237879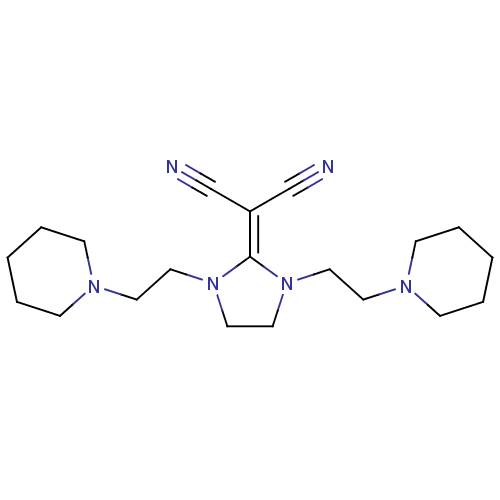 (2-(1,3-bis(2-(piperidin-1-yl)ethyl)imidazolidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237877 (2-(1-(2-((3-(dimethylamino)propyl)(ethyl)amino)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237877 (2-(1-(2-((3-(dimethylamino)propyl)(ethyl)amino)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220600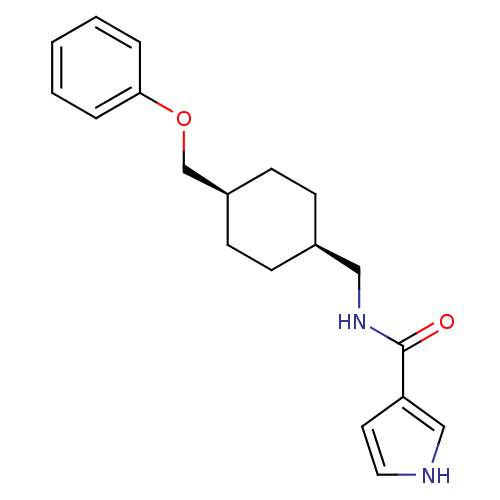 (CHEMBL398357 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220594 (3,5-dimethyl-N-(((1s,4s)-4-(phenoxymethyl)cyclohex...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220605 (6-oxo-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220593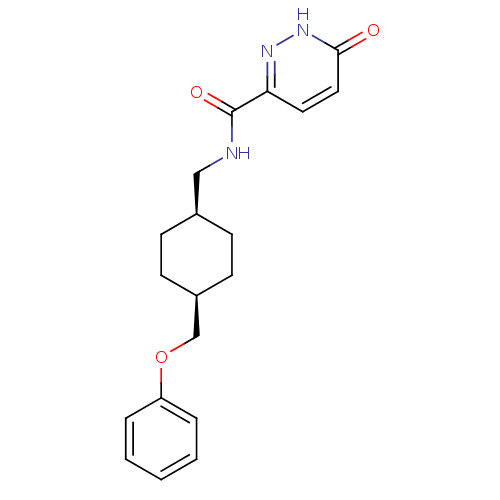 (6-hydroxy-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220609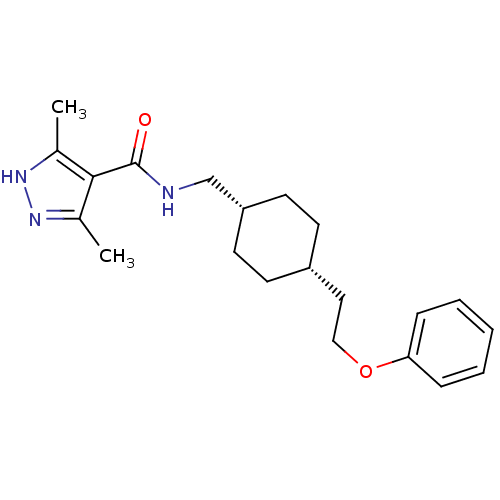 (3,5-dimethyl-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220604 (CHEMBL249297 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220596 (3-amino-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237876 (2-(1-(naphthalen-1-ylmethyl)-3-(3-(piperidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50231646 (3-(6-Chloro-naphthalene-2-sulfonyl)-1-[4-(2-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human thrombin after 30 mins | Bioorg Med Chem 16: 3125-40 (2008) Article DOI: 10.1016/j.bmc.2007.12.024 BindingDB Entry DOI: 10.7270/Q2VQ33J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1610 total ) | Next | Last >> |