

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Glutamate receptor ionotropic, NMDA 2B | ||
| Ligand | BDBM50220600 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_457343 (CHEMBL940934) | ||
| Ki | 84±n/a nM | ||
| Citation |  Kawai, M; Sakurada, I; Morita, A; Iwamuro, Y; Ando, K; Omura, H; Sakakibara, S; Masuda, T; Koike, H; Honma, T; Hattori, K; Takashima, T; Mizuno, K; Mizutani, M; Kawamura, M Structure-activity relationship study of novel NR2B-selective antagonists with arylamides to avoid reactive metabolites formation. Bioorg Med Chem Lett17:5537-42 (2007) [PubMed] Article Kawai, M; Sakurada, I; Morita, A; Iwamuro, Y; Ando, K; Omura, H; Sakakibara, S; Masuda, T; Koike, H; Honma, T; Hattori, K; Takashima, T; Mizuno, K; Mizutani, M; Kawamura, M Structure-activity relationship study of novel NR2B-selective antagonists with arylamides to avoid reactive metabolites formation. Bioorg Med Chem Lett17:5537-42 (2007) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Glutamate receptor ionotropic, NMDA 2B | |||
| Name: | Glutamate receptor ionotropic, NMDA 2B | ||
| Synonyms: | GluN2B | Glutamate [NMDA] receptor subunit epsilon 2 | Grin2b | N-methyl D-aspartate receptor subtype 2B | NMDA receptor subunit N2B (GluN2B) | NMDAR2B | NMDE2_RAT | NR2B | ||
| Type: | Protein | ||
| Mol. Mass.: | 166077.66 | ||
| Organism: | Rattus norvegicus (Rat) | ||
| Description: | Q00960 | ||
| Residue: | 1482 | ||
| Sequence: |
| ||
| BDBM50220600 | |||
| n/a | |||
| Name | BDBM50220600 | ||
| Synonyms: | CHEMBL398357 | N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)methyl)-1H-pyrrole-3-carboxamide | ||
| Type | Small organic molecule | ||
| Emp. Form. | C19H24N2O2 | ||
| Mol. Mass. | 312.4061 | ||
| SMILES | O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)c1cc[nH]c1 |wU:4.3,7.7,(-3.78,-5.13,;-3.78,-6.67,;-2.44,-7.44,;-1.11,-6.66,;.23,-7.43,;1.55,-6.65,;2.88,-7.43,;2.88,-8.97,;4.22,-9.74,;5.55,-8.97,;6.88,-9.74,;6.88,-11.28,;8.21,-12.05,;9.54,-11.28,;9.54,-9.74,;8.21,-8.97,;1.55,-9.73,;.23,-8.97,;-5.12,-7.44,;-6.52,-6.82,;-7.55,-7.97,;-6.78,-9.3,;-5.27,-8.97,)| | ||
| Structure | 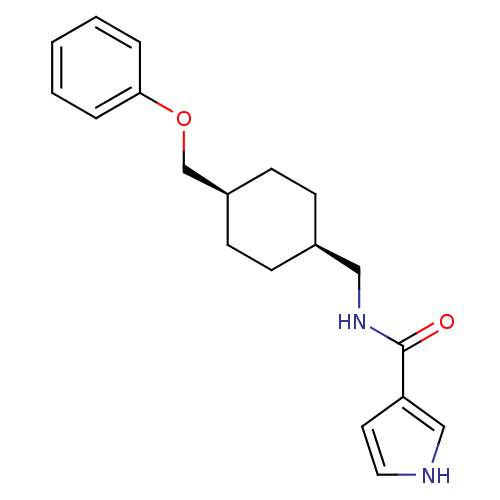
| ||