 Found 216 hits with Last Name = 'sakakibara' and Initial = 's'
Found 216 hits with Last Name = 'sakakibara' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220612
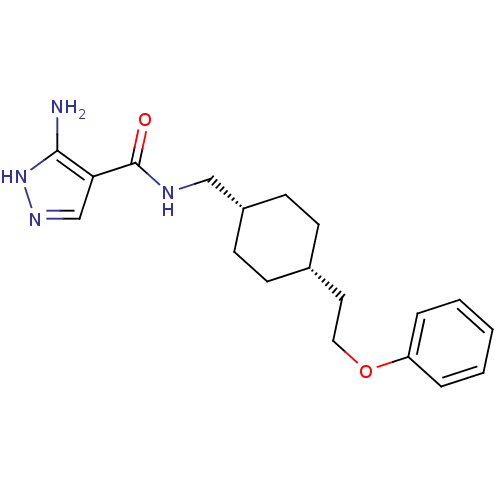
(3-amino-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)m...)Show SMILES Nc1[nH]ncc1C(=O)NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1 |wU:10.10,13.14,(18.38,-36.74,;18.71,-38.24,;17.68,-39.39,;18.45,-40.72,;19.96,-40.4,;20.12,-38.87,;21.45,-38.09,;21.44,-36.55,;22.78,-38.86,;24.11,-38.08,;25.45,-38.85,;25.45,-40.39,;26.78,-41.15,;28.11,-40.39,;29.44,-41.16,;30.77,-40.39,;32.11,-41.17,;33.44,-40.4,;34.77,-41.17,;36.11,-40.41,;36.11,-38.86,;34.77,-38.09,;33.44,-38.86,;28.11,-38.85,;26.78,-38.07,)| Show InChI InChI=1S/C19H26N4O2/c20-18-17(13-22-23-18)19(24)21-12-15-8-6-14(7-9-15)10-11-25-16-4-2-1-3-5-16/h1-5,13-15H,6-12H2,(H,21,24)(H3,20,22,23)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220606
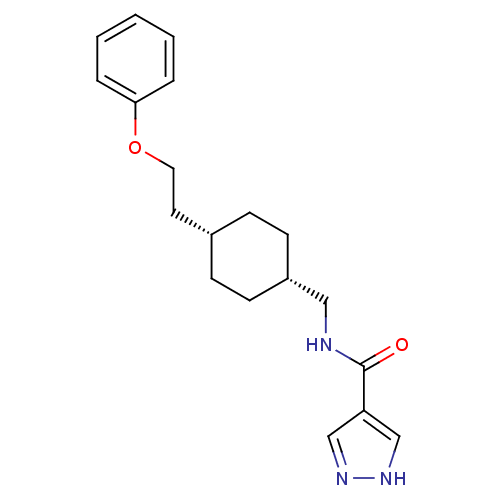
(CHEMBL249093 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1cn[nH]c1 |wU:4.3,7.7,(17.52,-15.71,;17.53,-17.25,;18.86,-18.01,;20.19,-17.24,;21.53,-18.01,;21.53,-19.55,;22.86,-20.31,;24.19,-19.55,;25.52,-20.32,;26.85,-19.55,;28.19,-20.32,;29.52,-19.55,;30.85,-20.33,;32.19,-19.56,;32.19,-18.02,;30.85,-17.25,;29.52,-18.02,;24.19,-18.01,;22.86,-17.23,;16.2,-18.02,;14.79,-17.4,;13.76,-18.55,;14.53,-19.88,;16.04,-19.55,)| Show InChI InChI=1S/C19H25N3O2/c23-19(17-13-21-22-14-17)20-12-16-8-6-15(7-9-16)10-11-24-18-4-2-1-3-5-18/h1-5,13-16H,6-12H2,(H,20,23)(H,21,22)/t15-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220611
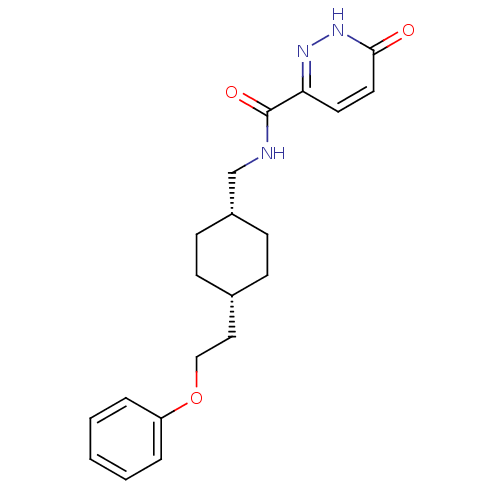
(6-hydroxy-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1ccc(=O)[nH]n1 |wU:4.3,7.7,(20.2,-34.76,;20.2,-36.3,;21.54,-37.06,;22.87,-36.29,;24.2,-37.05,;24.2,-38.59,;25.53,-39.36,;26.86,-38.59,;28.2,-39.37,;29.53,-38.6,;30.86,-39.37,;32.2,-38.6,;33.53,-39.38,;34.86,-38.61,;34.87,-37.07,;33.53,-36.3,;32.2,-37.07,;26.86,-37.05,;25.53,-36.28,;18.87,-37.07,;17.54,-36.3,;16.2,-37.07,;16.21,-38.61,;14.88,-39.39,;17.55,-39.38,;18.88,-38.6,)| Show InChI InChI=1S/C20H25N3O3/c24-19-11-10-18(22-23-19)20(25)21-14-16-8-6-15(7-9-16)12-13-26-17-4-2-1-3-5-17/h1-5,10-11,15-16H,6-9,12-14H2,(H,21,25)(H,23,24)/t15-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220592
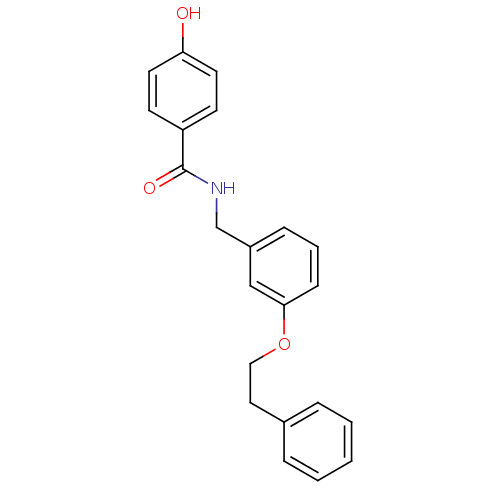
(CHEMBL248875 | N-(3-phenethoxybenzyl)-4-hydroxyben...)Show InChI InChI=1S/C22H21NO3/c24-20-11-9-19(10-12-20)22(25)23-16-18-7-4-8-21(15-18)26-14-13-17-5-2-1-3-6-17/h1-12,15,24H,13-14,16H2,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220608
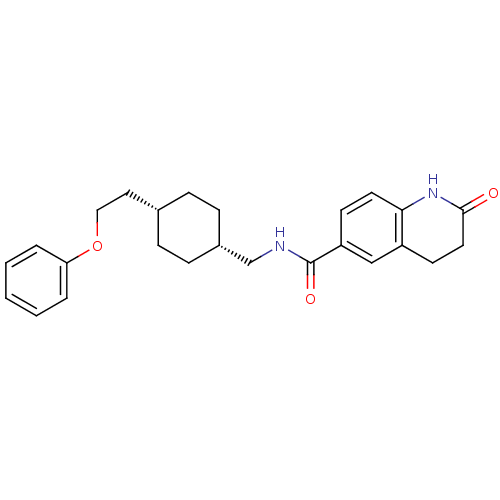
(2-oxo-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)met...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1ccc2NC(=O)CCc2c1 |wU:7.7,4.3,(21.1,-26.08,;21.1,-27.62,;22.44,-28.39,;23.77,-27.62,;25.11,-28.38,;25.11,-29.92,;26.44,-30.68,;27.76,-29.92,;29.1,-30.69,;30.43,-29.92,;31.76,-30.7,;33.1,-29.93,;34.43,-30.7,;35.77,-29.94,;35.77,-28.4,;34.43,-27.62,;33.1,-28.4,;27.76,-28.38,;26.44,-27.6,;19.77,-28.39,;19.78,-29.93,;18.46,-30.7,;17.11,-29.94,;15.78,-30.71,;14.45,-29.94,;13.11,-30.71,;14.45,-28.4,;15.78,-27.63,;17.11,-28.4,;18.44,-27.62,)| Show InChI InChI=1S/C25H30N2O3/c28-24-13-11-20-16-21(10-12-23(20)27-24)25(29)26-17-19-8-6-18(7-9-19)14-15-30-22-4-2-1-3-5-22/h1-5,10,12,16,18-19H,6-9,11,13-15,17H2,(H,26,29)(H,27,28)/t18-,19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220602
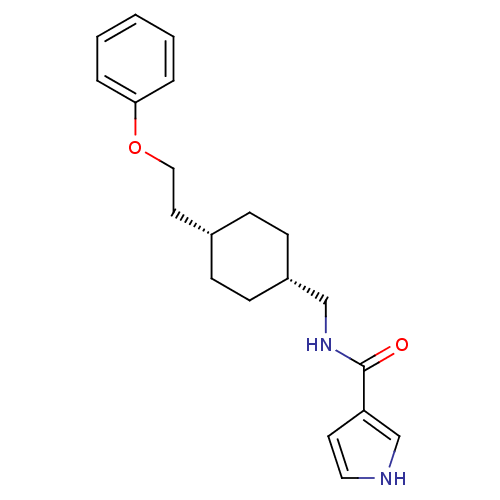
(CHEMBL249307 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1cc[nH]c1 |wU:4.3,7.7,(16.39,-6.18,;16.39,-7.72,;17.73,-8.49,;19.06,-7.71,;20.39,-8.48,;20.39,-10.02,;21.72,-10.78,;23.05,-10.02,;24.38,-10.79,;25.72,-10.02,;27.05,-10.79,;28.39,-10.03,;29.72,-10.8,;31.05,-10.03,;31.06,-8.49,;29.71,-7.72,;28.39,-8.49,;23.05,-8.48,;21.72,-7.7,;15.06,-8.49,;13.65,-7.87,;12.62,-9.02,;13.4,-10.35,;14.9,-10.03,)| Show InChI InChI=1S/C20H26N2O2/c23-20(18-10-12-21-15-18)22-14-17-8-6-16(7-9-17)11-13-24-19-4-2-1-3-5-19/h1-5,10,12,15-17,21H,6-9,11,13-14H2,(H,22,23)/t16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220603

(4-hydroxy-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)...)Show SMILES Oc1ccc(cc1)C(=O)NC[C@H]1CC[C@@H](COc2ccccc2)CC1 |wU:11.11,14.15,(-9.26,-9.14,;-7.93,-8.36,;-7.94,-6.82,;-6.61,-6.05,;-5.27,-6.82,;-5.26,-8.35,;-6.59,-9.13,;-3.94,-6.05,;-3.95,-4.51,;-2.61,-6.81,;-1.27,-6.04,;.06,-6.81,;1.39,-6.03,;2.72,-6.81,;2.72,-8.35,;4.05,-9.12,;5.39,-8.35,;6.72,-9.12,;6.71,-10.66,;8.04,-11.43,;9.38,-10.66,;9.38,-9.11,;8.05,-8.35,;1.39,-9.11,;.06,-8.35,)| Show InChI InChI=1S/C21H25NO3/c23-19-12-10-18(11-13-19)21(24)22-14-16-6-8-17(9-7-16)15-25-20-4-2-1-3-5-20/h1-5,10-13,16-17,23H,6-9,14-15H2,(H,22,24)/t16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220595
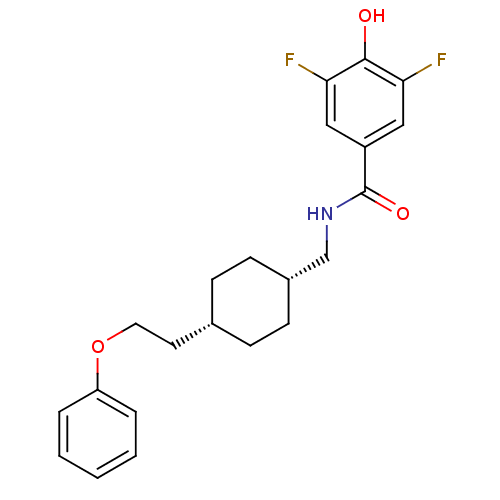
(3,5-difluoro-4-hydroxy-N-(((1s,4s)-4-(2-phenoxyeth...)Show SMILES Oc1c(F)cc(cc1F)C(=O)NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1 |wU:13.13,16.17,(13.63,-21.21,;14.96,-20.43,;14.96,-18.89,;13.62,-18.13,;16.29,-18.12,;17.63,-18.89,;17.63,-20.42,;16.31,-21.2,;16.32,-22.74,;18.96,-18.12,;18.95,-16.58,;20.29,-18.88,;21.62,-18.11,;22.96,-18.88,;22.96,-20.42,;24.29,-21.18,;25.62,-20.42,;26.95,-21.19,;28.29,-20.42,;29.62,-21.19,;30.95,-20.42,;32.29,-21.2,;33.62,-20.43,;33.62,-18.89,;32.28,-18.12,;30.95,-18.89,;25.62,-18.88,;24.29,-18.1,)| Show InChI InChI=1S/C22H25F2NO3/c23-19-12-17(13-20(24)21(19)26)22(27)25-14-16-8-6-15(7-9-16)10-11-28-18-4-2-1-3-5-18/h1-5,12-13,15-16,26H,6-11,14H2,(H,25,27)/t15-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220591
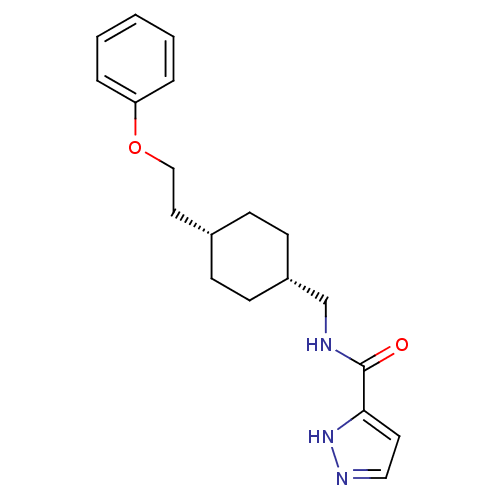
(CHEMBL249909 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1ccn[nH]1 |wU:4.3,7.7,(17.4,-25.44,;17.41,-26.98,;18.74,-27.74,;20.08,-26.97,;21.41,-27.74,;21.41,-29.28,;22.74,-30.04,;24.07,-29.28,;25.4,-30.05,;26.74,-29.28,;28.07,-30.05,;29.41,-29.28,;30.74,-30.06,;32.07,-29.29,;32.08,-27.75,;30.73,-26.98,;29.4,-27.75,;24.07,-27.74,;22.74,-26.96,;16.08,-27.75,;14.67,-27.13,;13.64,-28.28,;14.42,-29.61,;15.92,-29.28,)| Show InChI InChI=1S/C19H25N3O2/c23-19(18-10-12-21-22-18)20-14-16-8-6-15(7-9-16)11-13-24-17-4-2-1-3-5-17/h1-5,10,12,15-16H,6-9,11,13-14H2,(H,20,23)(H,21,22)/t15-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220598
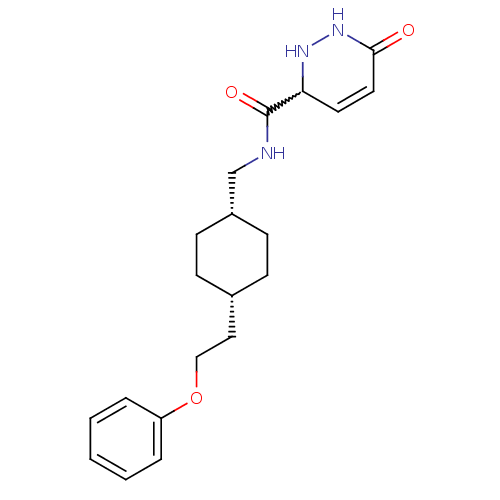
(6-oxo-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)met...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)C1NNC(=O)C=C1 |w:19.20,wU:4.3,7.7,c:26,(18.91,-43.44,;18.92,-44.98,;20.25,-45.74,;21.59,-44.97,;22.92,-45.73,;22.92,-47.27,;24.25,-48.04,;25.58,-47.27,;26.91,-48.05,;28.25,-47.28,;29.58,-48.05,;30.91,-47.28,;32.25,-48.06,;33.58,-47.29,;33.58,-45.75,;32.24,-44.98,;30.91,-45.75,;25.58,-45.73,;24.25,-44.96,;17.59,-45.75,;17.6,-47.29,;16.26,-48.06,;14.92,-47.29,;13.59,-48.05,;14.92,-45.75,;16.25,-44.98,)| Show InChI InChI=1S/C20H27N3O3/c24-19-11-10-18(22-23-19)20(25)21-14-16-8-6-15(7-9-16)12-13-26-17-4-2-1-3-5-17/h1-5,10-11,15-16,18,22H,6-9,12-14H2,(H,21,25)(H,23,24)/t15-,16+,18? | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220599

(3,5-difluoro-4-hydroxy-N-(((1s,4s)-4-(phenoxymethy...)Show SMILES Oc1c(F)cc(cc1F)C(=O)NC[C@H]1CC[C@@H](COc2ccccc2)CC1 |wU:13.13,16.17,(-9.57,-19.5,;-8.24,-18.72,;-8.24,-17.18,;-9.58,-16.42,;-6.91,-16.41,;-5.57,-17.18,;-5.57,-18.71,;-6.89,-19.49,;-6.88,-21.03,;-4.24,-16.41,;-4.25,-14.87,;-2.91,-17.17,;-1.58,-16.4,;-.24,-17.17,;1.09,-16.39,;2.42,-17.17,;2.42,-18.71,;3.75,-19.48,;5.08,-18.71,;6.42,-19.48,;6.41,-21.02,;7.74,-21.79,;9.08,-21.02,;9.07,-19.47,;7.74,-18.71,;1.09,-19.47,;-.24,-18.71,)| Show InChI InChI=1S/C21H23F2NO3/c22-18-10-16(11-19(23)20(18)25)21(26)24-12-14-6-8-15(9-7-14)13-27-17-4-2-1-3-5-17/h1-5,10-11,14-15,25H,6-9,12-13H2,(H,24,26)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220610

(CHEMBL250108 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)c1cn[nH]c1 |wU:4.3,7.7,(-4.35,-14.31,;-4.35,-15.85,;-3.01,-16.61,;-1.68,-15.84,;-.34,-16.61,;.99,-15.83,;2.32,-16.61,;2.32,-18.15,;3.65,-18.92,;4.98,-18.15,;6.32,-18.92,;6.31,-20.46,;7.64,-21.23,;8.98,-20.46,;8.97,-18.91,;7.64,-18.15,;.99,-18.91,;-.34,-18.15,;-5.68,-16.62,;-7.09,-16,;-8.12,-17.14,;-7.35,-18.47,;-5.84,-18.15,)| Show InChI InChI=1S/C18H23N3O2/c22-18(16-11-20-21-12-16)19-10-14-6-8-15(9-7-14)13-23-17-4-2-1-3-5-17/h1-5,11-12,14-15H,6-10,13H2,(H,19,22)(H,20,21)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220597
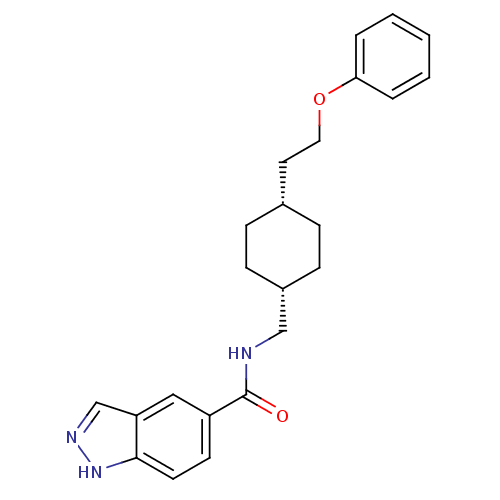
(CHEMBL398356 | N-(((1s,4s)-4-(2-phenoxyethyl)cyclo...)Show SMILES O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)c1ccc2[nH]ncc2c1 |wU:4.3,7.7,(-1.62,4.65,;-1.62,3.11,;-.28,2.35,;1.05,3.12,;2.39,2.36,;2.39,.82,;3.72,.05,;5.05,.82,;6.38,.04,;7.71,.81,;9.05,.04,;10.38,.81,;11.71,.03,;13.05,.8,;13.05,2.34,;11.71,3.11,;10.38,2.34,;5.05,2.36,;3.72,3.13,;-2.95,2.34,;-2.94,.81,;-4.26,.03,;-5.61,.8,;-7.08,.33,;-7.99,1.58,;-7.07,2.83,;-5.6,2.35,;-4.28,3.11,)| Show InChI InChI=1S/C23H27N3O2/c27-23(19-10-11-22-20(14-19)16-25-26-22)24-15-18-8-6-17(7-9-18)12-13-28-21-4-2-1-3-5-21/h1-5,10-11,14,16-18H,6-9,12-13,15H2,(H,24,27)(H,25,26)/t17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220601
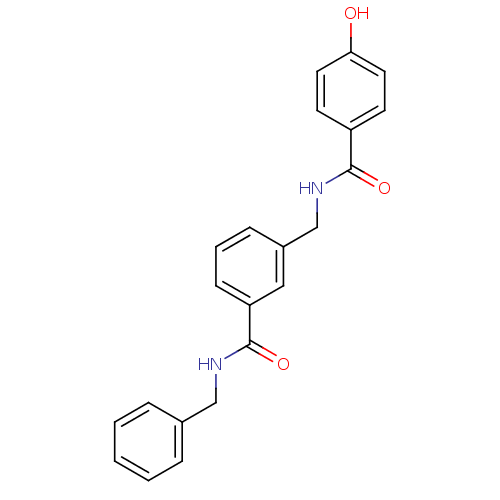
(CHEMBL400917 | N-benzyl-3-{[(4-hydroxybenzoyl)amin...)Show SMILES Oc1ccc(cc1)C(=O)NCc1cccc(c1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C22H20N2O3/c25-20-11-9-18(10-12-20)21(26)24-15-17-7-4-8-19(13-17)22(27)23-14-16-5-2-1-3-6-16/h1-13,25H,14-15H2,(H,23,27)(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220607
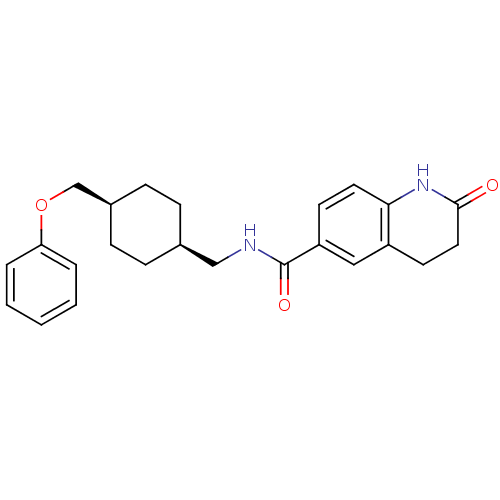
(2-oxo-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)meth...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)c1ccc2NC(=O)CCc2c1 |wU:4.3,7.7,(-2.62,-24.66,;-2.61,-26.2,;-1.28,-26.97,;.06,-26.19,;1.39,-26.96,;2.72,-26.18,;4.05,-26.96,;4.05,-28.5,;5.38,-29.27,;6.72,-28.5,;8.05,-29.27,;8.04,-30.81,;9.37,-31.58,;10.71,-30.81,;10.71,-29.27,;9.38,-28.5,;2.72,-29.26,;1.39,-28.5,;-3.94,-26.97,;-3.93,-28.5,;-5.26,-29.28,;-6.6,-28.52,;-7.93,-29.3,;-9.27,-28.53,;-10.6,-29.31,;-9.28,-26.99,;-7.94,-26.22,;-6.61,-26.97,;-5.28,-26.2,)| Show InChI InChI=1S/C24H28N2O3/c27-23-13-11-19-14-20(10-12-22(19)26-23)24(28)25-15-17-6-8-18(9-7-17)16-29-21-4-2-1-3-5-21/h1-5,10,12,14,17-18H,6-9,11,13,15-16H2,(H,25,28)(H,26,27)/t17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220590
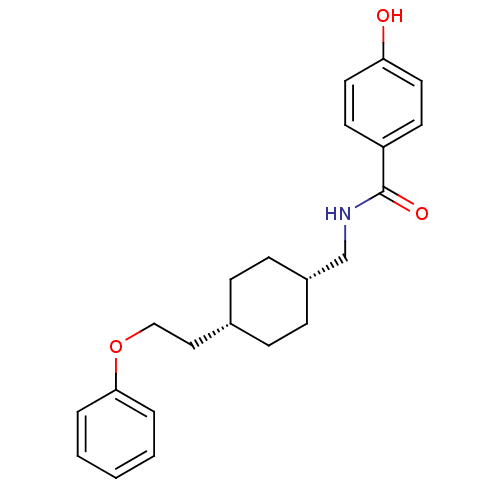
(4-hydroxy-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl...)Show SMILES Oc1ccc(cc1)C(=O)NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1 |wU:11.11,14.15,(12.9,-11.25,;14.23,-10.47,;14.23,-8.93,;15.56,-8.16,;16.89,-8.93,;16.9,-10.46,;15.58,-11.24,;18.23,-8.16,;18.22,-6.62,;19.56,-8.92,;20.89,-8.15,;22.23,-8.91,;22.23,-10.45,;23.56,-11.22,;24.89,-10.45,;26.22,-11.23,;27.55,-10.46,;28.89,-11.23,;30.22,-10.46,;31.55,-11.24,;32.89,-10.47,;32.89,-8.93,;31.55,-8.16,;30.22,-8.93,;24.89,-8.91,;23.56,-8.14,)| Show InChI InChI=1S/C22H27NO3/c24-20-12-10-19(11-13-20)22(25)23-16-18-8-6-17(7-9-18)14-15-26-21-4-2-1-3-5-21/h1-5,10-13,17-18,24H,6-9,14-16H2,(H,23,25)/t17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220600
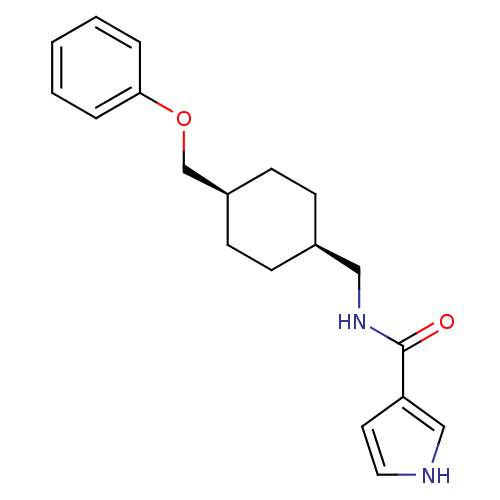
(CHEMBL398357 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)c1cc[nH]c1 |wU:4.3,7.7,(-3.78,-5.13,;-3.78,-6.67,;-2.44,-7.44,;-1.11,-6.66,;.23,-7.43,;1.55,-6.65,;2.88,-7.43,;2.88,-8.97,;4.22,-9.74,;5.55,-8.97,;6.88,-9.74,;6.88,-11.28,;8.21,-12.05,;9.54,-11.28,;9.54,-9.74,;8.21,-8.97,;1.55,-9.73,;.23,-8.97,;-5.12,-7.44,;-6.52,-6.82,;-7.55,-7.97,;-6.78,-9.3,;-5.27,-8.97,)| Show InChI InChI=1S/C19H24N2O2/c22-19(17-10-11-20-13-17)21-12-15-6-8-16(9-7-15)14-23-18-4-2-1-3-5-18/h1-5,10-11,13,15-16,20H,6-9,12,14H2,(H,21,22)/t15-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220594

(3,5-dimethyl-N-(((1s,4s)-4-(phenoxymethyl)cyclohex...)Show SMILES Cc1n[nH]c(C)c1C(=O)NC[C@H]1CC[C@@H](COc2ccccc2)CC1 |wU:11.11,14.15,(-5.39,-42.57,;-5.07,-44.07,;-6.1,-45.22,;-5.32,-46.55,;-3.82,-46.23,;-2.67,-47.25,;-3.66,-44.7,;-2.32,-43.93,;-2.33,-42.39,;-.99,-44.69,;.34,-43.92,;1.68,-44.68,;3.01,-43.91,;4.34,-44.68,;4.34,-46.22,;5.67,-47,;7.01,-46.23,;8.34,-47,;8.33,-48.54,;9.66,-49.31,;11,-48.54,;11,-46.99,;9.66,-46.23,;3.01,-46.99,;1.68,-46.22,)| Show InChI InChI=1S/C20H27N3O2/c1-14-19(15(2)23-22-14)20(24)21-12-16-8-10-17(11-9-16)13-25-18-6-4-3-5-7-18/h3-7,16-17H,8-13H2,1-2H3,(H,21,24)(H,22,23)/t16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220605

(6-oxo-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)meth...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)C1NNC(=O)C=C1 |w:18.19,wU:4.3,7.7,c:25,(-3.75,-42.93,;-3.75,-44.47,;-2.41,-45.24,;-1.08,-44.46,;.26,-45.23,;1.59,-44.45,;2.91,-45.23,;2.91,-46.77,;4.25,-47.54,;5.58,-46.77,;6.91,-47.54,;6.91,-49.08,;8.24,-49.85,;9.57,-49.08,;9.57,-47.54,;8.24,-46.77,;1.59,-47.53,;.26,-46.77,;-5.08,-45.25,;-5.07,-46.79,;-6.41,-47.56,;-7.75,-46.79,;-9.08,-47.56,;-7.74,-45.25,;-6.42,-44.48,)| Show InChI InChI=1S/C19H25N3O3/c23-18-11-10-17(21-22-18)19(24)20-12-14-6-8-15(9-7-14)13-25-16-4-2-1-3-5-16/h1-5,10-11,14-15,17,21H,6-9,12-13H2,(H,20,24)(H,22,23)/t14-,15+,17? | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220596

(3-amino-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)me...)Show SMILES Nc1[nH]ncc1C(=O)NC[C@H]1CC[C@@H](COc2ccccc2)CC1 |wU:10.10,13.14,(-6.27,-33.66,;-5.94,-35.16,;-6.97,-36.31,;-6.19,-37.64,;-4.69,-37.31,;-4.53,-35.78,;-3.19,-35.01,;-3.2,-33.47,;-1.86,-35.78,;-.53,-35,;.81,-35.77,;2.14,-34.99,;3.47,-35.77,;3.47,-37.31,;4.8,-38.08,;6.13,-37.31,;7.47,-38.09,;7.46,-39.62,;8.79,-40.39,;10.13,-39.63,;10.12,-38.08,;8.79,-37.31,;2.14,-38.07,;.81,-37.31,)| Show InChI InChI=1S/C18H24N4O2/c19-17-16(11-21-22-17)18(23)20-10-13-6-8-14(9-7-13)12-24-15-4-2-1-3-5-15/h1-5,11,13-14H,6-10,12H2,(H,20,23)(H3,19,21,22)/t13-,14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220604

(CHEMBL249297 | N-(((1s,4s)-4-(phenoxymethyl)cycloh...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)c1ccn[nH]1 |wU:4.3,7.7,(-3.28,-24.38,;-3.28,-25.92,;-1.94,-26.69,;-.61,-25.91,;.72,-26.68,;2.05,-25.9,;3.38,-26.68,;3.38,-28.22,;4.71,-28.99,;6.05,-28.22,;7.38,-28.99,;7.37,-30.53,;8.7,-31.3,;10.04,-30.53,;10.04,-28.99,;8.71,-28.22,;2.05,-28.98,;.72,-28.22,;-4.62,-26.69,;-6.03,-26.07,;-7.05,-27.22,;-6.28,-28.55,;-4.77,-28.22,)| Show InChI InChI=1S/C18H23N3O2/c22-18(17-10-11-20-21-17)19-12-14-6-8-15(9-7-14)13-23-16-4-2-1-3-5-16/h1-5,10-11,14-15H,6-9,12-13H2,(H,19,22)(H,20,21)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220593
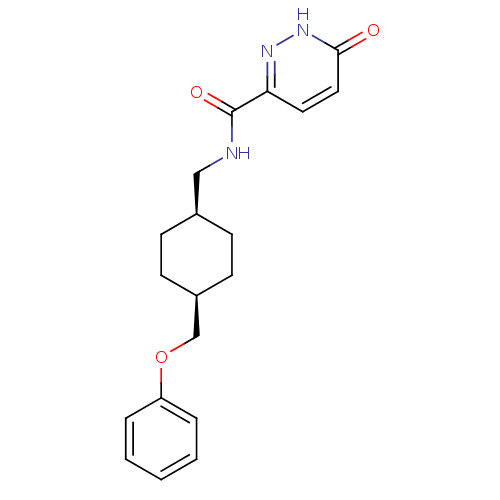
(6-hydroxy-N-(((1s,4s)-4-(phenoxymethyl)cyclohexyl)...)Show SMILES O=C(NC[C@H]1CC[C@@H](COc2ccccc2)CC1)c1ccc(=O)[nH]n1 |wU:4.3,7.7,(-3.93,-34.59,;-3.93,-36.13,;-2.59,-36.89,;-1.26,-36.12,;.08,-36.89,;1.41,-36.11,;2.74,-36.89,;2.74,-38.43,;4.07,-39.2,;5.4,-38.43,;6.74,-39.2,;6.73,-40.74,;8.06,-41.51,;9.4,-40.74,;9.39,-39.19,;8.06,-38.43,;1.41,-39.19,;.08,-38.43,;-5.26,-36.9,;-6.59,-36.13,;-7.92,-36.9,;-7.92,-38.44,;-9.25,-39.22,;-6.57,-39.21,;-5.25,-38.43,)| Show InChI InChI=1S/C19H23N3O3/c23-18-11-10-17(21-22-18)19(24)20-12-14-6-8-15(9-7-14)13-25-16-4-2-1-3-5-16/h1-5,10-11,14-15H,6-9,12-13H2,(H,20,24)(H,22,23)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220609
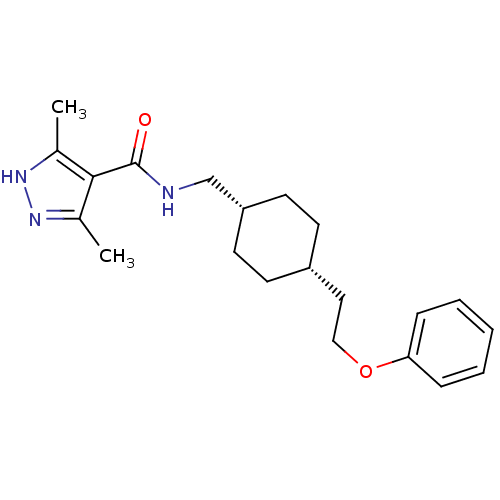
(3,5-dimethyl-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohe...)Show SMILES Cc1n[nH]c(C)c1C(=O)NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1 |wU:11.11,14.15,(19.66,-44.61,;19.98,-46.12,;18.95,-47.26,;19.73,-48.59,;21.23,-48.27,;22.38,-49.3,;21.39,-46.74,;22.72,-45.96,;22.72,-44.42,;24.06,-46.73,;25.39,-45.96,;26.72,-46.72,;26.72,-48.26,;28.05,-49.02,;29.38,-48.26,;30.72,-49.03,;32.05,-48.26,;33.38,-49.04,;34.72,-48.27,;36.05,-49.04,;37.38,-48.28,;37.39,-46.74,;36.05,-45.96,;34.72,-46.74,;29.38,-46.72,;28.05,-45.94,)| Show InChI InChI=1S/C21H29N3O2/c1-15-20(16(2)24-23-15)21(25)22-14-18-10-8-17(9-11-18)12-13-26-19-6-4-3-5-7-19/h3-7,17-18H,8-14H2,1-2H3,(H,22,25)(H,23,24)/t17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane |
Bioorg Med Chem Lett 17: 5537-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.033
BindingDB Entry DOI: 10.7270/Q2M908D0 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149846
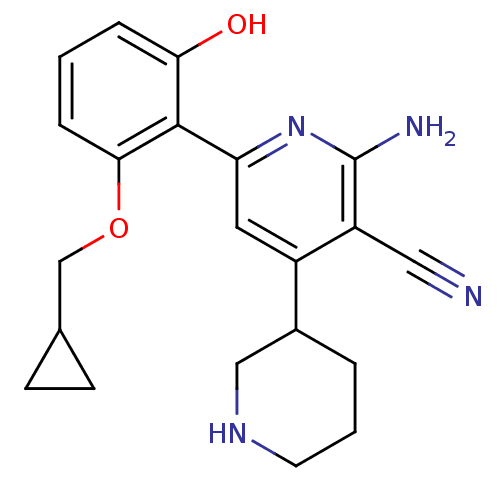
(2''-Amino-6''-(2-cyclopropylmethoxy-6-hydroxy-phen...)Show SMILES Nc1nc(cc(C2CCCNC2)c1C#N)-c1c(O)cccc1OCC1CC1 Show InChI InChI=1S/C21H24N4O2/c22-10-16-15(14-3-2-8-24-11-14)9-17(25-21(16)23)20-18(26)4-1-5-19(20)27-12-13-6-7-13/h1,4-5,9,13-14,24,26H,2-3,6-8,11-12H2,(H2,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149839
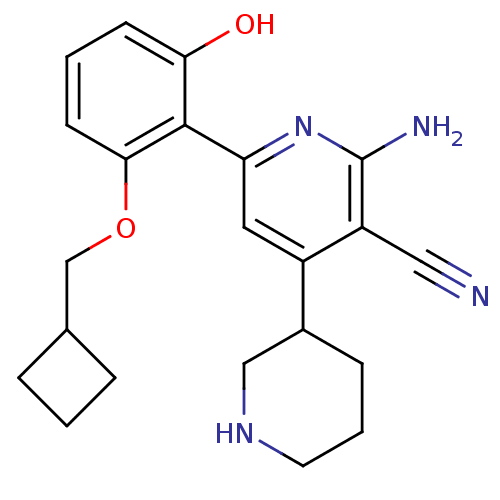
(2''-Amino-6''-(2-cyclobutylmethoxy-6-hydroxy-pheny...)Show SMILES Nc1nc(cc(C2CCCNC2)c1C#N)-c1c(O)cccc1OCC1CCC1 Show InChI InChI=1S/C22H26N4O2/c23-11-17-16(15-6-3-9-25-12-15)10-18(26-22(17)24)21-19(27)7-2-8-20(21)28-13-14-4-1-5-14/h2,7-8,10,14-15,25,27H,1,3-6,9,12-13H2,(H2,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149832

(2''-Amino-6''-(2-hydroxy-6-propoxy-phenyl)-1,2,3,4...)Show InChI InChI=1S/C20H24N4O2/c1-2-9-26-18-7-3-6-17(25)19(18)16-10-14(13-5-4-8-23-12-13)15(11-21)20(22)24-16/h3,6-7,10,13,23,25H,2,4-5,8-9,12H2,1H3,(H2,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149833

(2''-Amino-6''-(2-hydroxy-6-isobutoxy-phenyl)-1,2,3...)Show SMILES CC(C)COc1cccc(O)c1-c1cc(C2CCCNC2)c(C#N)c(N)n1 Show InChI InChI=1S/C21H26N4O2/c1-13(2)12-27-19-7-3-6-18(26)20(19)17-9-15(14-5-4-8-24-11-14)16(10-22)21(23)25-17/h3,6-7,9,13-14,24,26H,4-5,8,11-12H2,1-2H3,(H2,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149838
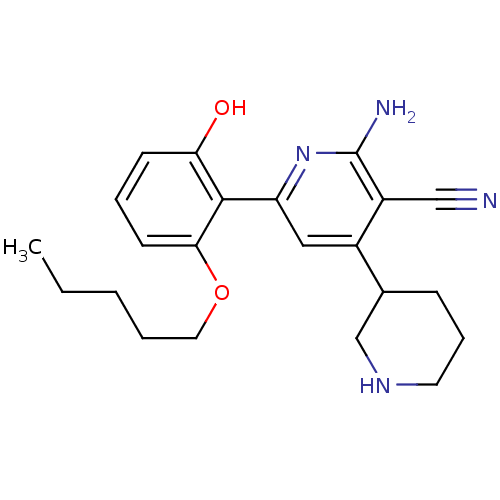
(2''-Amino-6''-(2-hydroxy-6-pentyloxy-phenyl)-1,2,3...)Show SMILES CCCCCOc1cccc(O)c1-c1cc(C2CCCNC2)c(C#N)c(N)n1 Show InChI InChI=1S/C22H28N4O2/c1-2-3-4-11-28-20-9-5-8-19(27)21(20)18-12-16(15-7-6-10-25-14-15)17(13-23)22(24)26-18/h5,8-9,12,15,25,27H,2-4,6-7,10-11,14H2,1H3,(H2,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149825
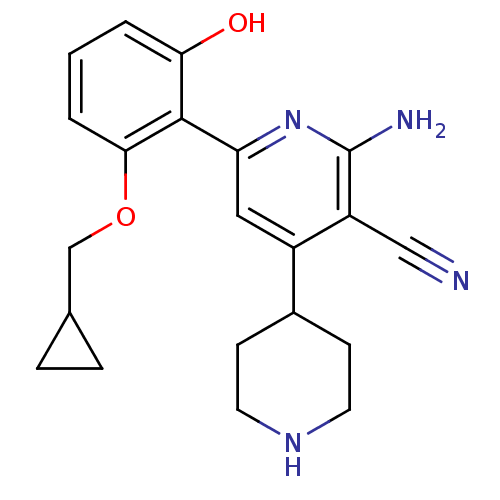
(2-Amino-6-(2-cyclopropylmethoxy-6-hydroxy-phenyl)-...)Show SMILES Nc1nc(cc(C2CCNCC2)c1C#N)-c1c(O)cccc1OCC1CC1 Show InChI InChI=1S/C21H24N4O2/c22-11-16-15(14-6-8-24-9-7-14)10-17(25-21(16)23)20-18(26)2-1-3-19(20)27-12-13-4-5-13/h1-3,10,13-14,24,26H,4-9,12H2,(H2,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149843

(2''-Amino-6''-(2-benzyloxy-6-hydroxy-phenyl)-1,2,3...)Show SMILES Nc1nc(cc(C2CCCNC2)c1C#N)-c1c(O)cccc1OCc1ccccc1 Show InChI InChI=1S/C24H24N4O2/c25-13-19-18(17-8-5-11-27-14-17)12-20(28-24(19)26)23-21(29)9-4-10-22(23)30-15-16-6-2-1-3-7-16/h1-4,6-7,9-10,12,17,27,29H,5,8,11,14-15H2,(H2,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149828

(2-Amino-6-(2-cyclobutylmethoxy-6-hydroxy-phenyl)-1...)Show SMILES Nc1nc(cc(C2CCNCC2)c1C#N)-c1c(O)cccc1OCC1CCC1 Show InChI InChI=1S/C22H26N4O2/c23-12-17-16(15-7-9-25-10-8-15)11-18(26-22(17)24)21-19(27)5-2-6-20(21)28-13-14-3-1-4-14/h2,5-6,11,14-15,25,27H,1,3-4,7-10,13H2,(H2,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149852
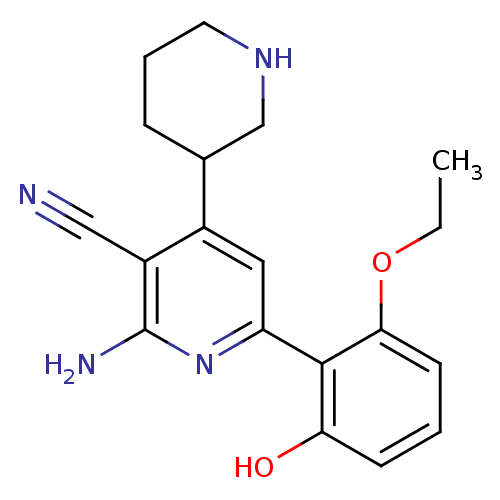
(2''-Amino-6''-(2-ethoxy-6-hydroxy-phenyl)-1,2,3,4,...)Show InChI InChI=1S/C19H22N4O2/c1-2-25-17-7-3-6-16(24)18(17)15-9-13(12-5-4-8-22-11-12)14(10-20)19(21)23-15/h3,6-7,9,12,22,24H,2,4-5,8,11H2,1H3,(H2,21,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149831
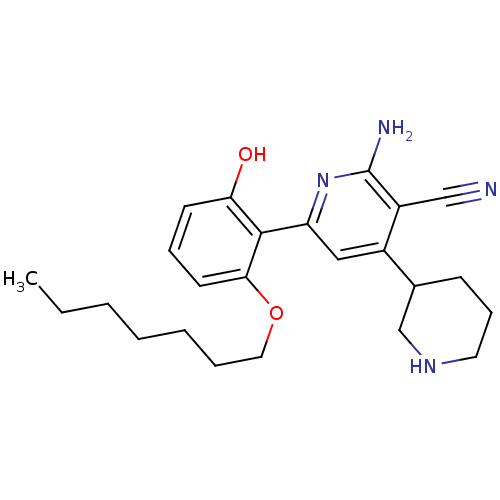
(2''-Amino-6''-(2-heptyloxy-6-hydroxy-phenyl)-1,2,3...)Show SMILES CCCCCCCOc1cccc(O)c1-c1cc(C2CCCNC2)c(C#N)c(N)n1 Show InChI InChI=1S/C24H32N4O2/c1-2-3-4-5-6-13-30-22-11-7-10-21(29)23(22)20-14-18(17-9-8-12-27-16-17)19(15-25)24(26)28-20/h7,10-11,14,17,27,29H,2-6,8-9,12-13,16H2,1H3,(H2,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149842

(2''-Amino-6''-(2,6-dihydroxy-phenyl)-1,2,3,4,5,6-h...)Show InChI InChI=1S/C17H18N4O2/c18-8-12-11(10-3-2-6-20-9-10)7-13(21-17(12)19)16-14(22)4-1-5-15(16)23/h1,4-5,7,10,20,22-23H,2-3,6,9H2,(H2,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149837
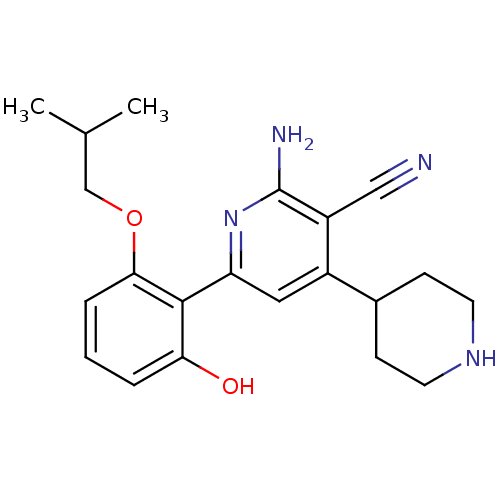
(2-Amino-6-(2-hydroxy-6-isobutoxy-phenyl)-1'',2'',3...)Show SMILES CC(C)COc1cccc(O)c1-c1cc(C2CCNCC2)c(C#N)c(N)n1 Show InChI InChI=1S/C21H26N4O2/c1-13(2)12-27-19-5-3-4-18(26)20(19)17-10-15(14-6-8-24-9-7-14)16(11-22)21(23)25-17/h3-5,10,13-14,24,26H,6-9,12H2,1-2H3,(H2,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149850
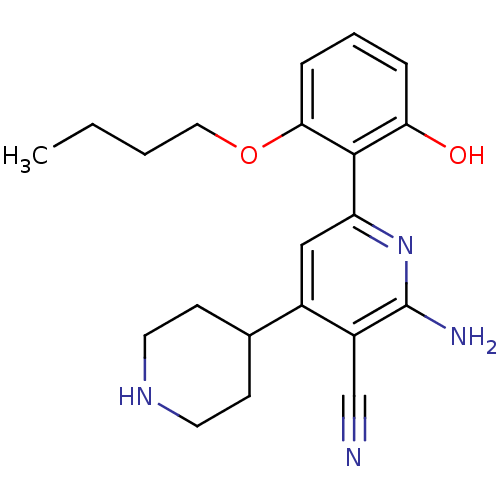
(2-Amino-6-(2-butoxy-6-hydroxy-phenyl)-1'',2'',3'',...)Show SMILES CCCCOc1cccc(O)c1-c1cc(C2CCNCC2)c(C#N)c(N)n1 Show InChI InChI=1S/C21H26N4O2/c1-2-3-11-27-19-6-4-5-18(26)20(19)17-12-15(14-7-9-24-10-8-14)16(13-22)21(23)25-17/h4-6,12,14,24,26H,2-3,7-11H2,1H3,(H2,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165518
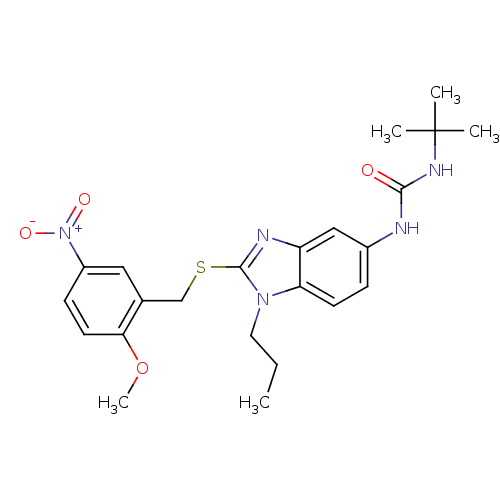
(1-tert-Butyl-3-[2-(2-methoxy-5-nitro-benzylsulfany...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)ccc12 Show InChI InChI=1S/C23H29N5O4S/c1-6-11-27-19-9-7-16(24-21(29)26-23(2,3)4)13-18(19)25-22(27)33-14-15-12-17(28(30)31)8-10-20(15)32-5/h7-10,12-13H,6,11,14H2,1-5H3,(H2,24,26,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165530

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCCCN(C)C)c12 Show InChI InChI=1S/C29H41N7O5S/c1-8-13-35-25-22(26(37)30-12-9-14-34(5)6)16-20(31-27(38)33-29(2,3)4)17-23(25)32-28(35)42-18-19-15-21(36(39)40)10-11-24(19)41-7/h10-11,15-17H,8-9,12-14,18H2,1-7H3,(H,30,37)(H2,31,33,38) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149826
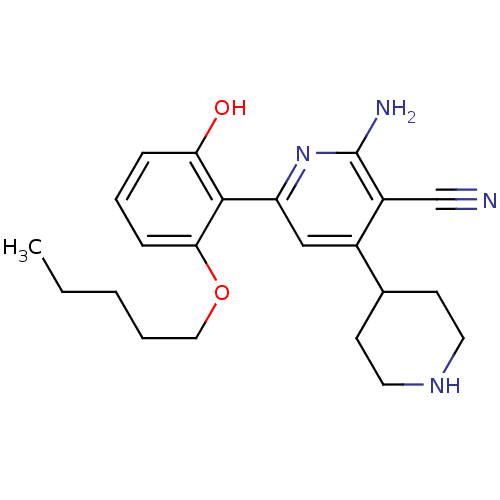
(2-Amino-6-(2-hydroxy-6-pentyloxy-phenyl)-1'',2'',3...)Show SMILES CCCCCOc1cccc(O)c1-c1cc(C2CCNCC2)c(C#N)c(N)n1 Show InChI InChI=1S/C22H28N4O2/c1-2-3-4-12-28-20-7-5-6-19(27)21(20)18-13-16(15-8-10-25-11-9-15)17(14-23)22(24)26-18/h5-7,13,15,25,27H,2-4,8-12H2,1H3,(H2,24,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149812
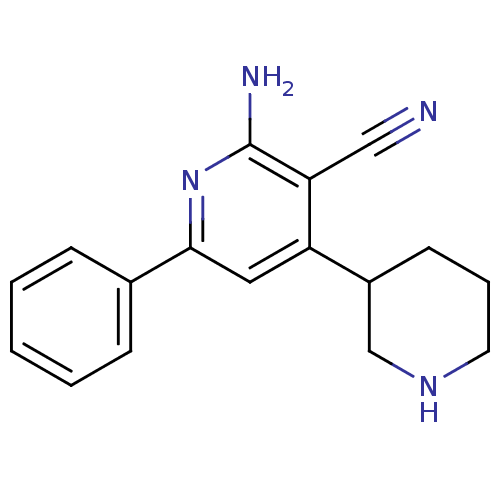
(2''-Amino-6''-phenyl-1,2,3,4,5,6-hexahydro-[3,4'']...)Show InChI InChI=1S/C17H18N4/c18-10-15-14(13-7-4-8-20-11-13)9-16(21-17(15)19)12-5-2-1-3-6-12/h1-3,5-6,9,13,20H,4,7-8,11H2,(H2,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human I-kappa-B-kinase beta |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165553
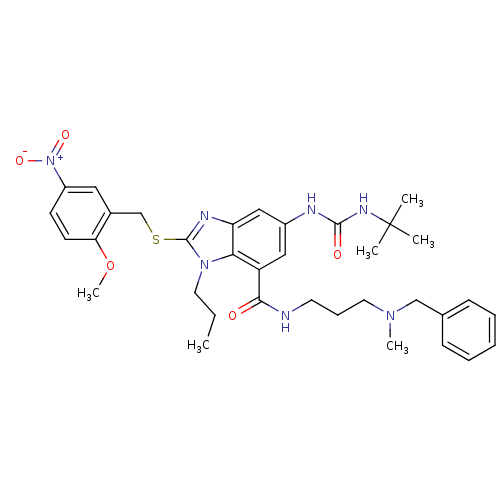
(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NCCCN(C)Cc3ccccc3)c12 Show InChI InChI=1S/C35H45N7O5S/c1-7-17-41-31-28(32(43)36-16-11-18-40(5)22-24-12-9-8-10-13-24)20-26(37-33(44)39-35(2,3)4)21-29(31)38-34(41)48-23-25-19-27(42(45)46)14-15-30(25)47-6/h8-10,12-15,19-21H,7,11,16-18,22-23H2,1-6H3,(H,36,43)(H2,37,39,44) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165538

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(O)=O)c12 Show InChI InChI=1S/C24H29N5O6S/c1-6-9-28-20-17(21(30)31)11-15(25-22(32)27-24(2,3)4)12-18(20)26-23(28)36-13-14-10-16(29(33)34)7-8-19(14)35-5/h7-8,10-12H,6,9,13H2,1-5H3,(H,30,31)(H2,25,27,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50165528

(6-(3-tert-Butyl-ureido)-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)cc(C(=O)NC)c12 Show InChI InChI=1S/C25H32N6O5S/c1-7-10-30-21-18(22(32)26-5)12-16(27-23(33)29-25(2,3)4)13-19(21)28-24(30)37-14-15-11-17(31(34)35)8-9-20(15)36-6/h8-9,11-13H,7,10,14H2,1-6H3,(H,26,32)(H2,27,29,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50165539
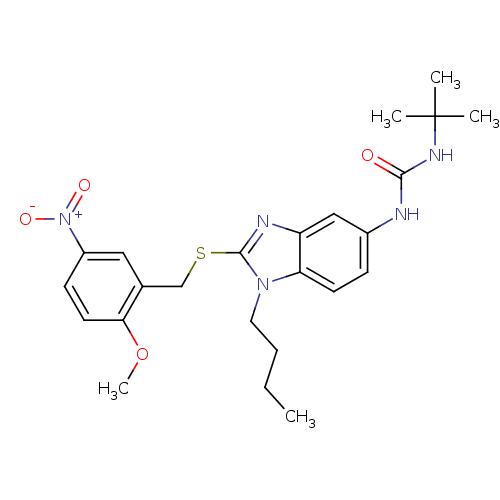
(1-tert-Butyl-3-[1-butyl-2-(2-methoxy-5-nitro-benzy...)Show SMILES CCCCn1c(SCc2cc(ccc2OC)[N+]([O-])=O)nc2cc(NC(=O)NC(C)(C)C)ccc12 Show InChI InChI=1S/C24H31N5O4S/c1-6-7-12-28-20-10-8-17(25-22(30)27-24(2,3)4)14-19(20)26-23(28)34-15-16-13-18(29(31)32)9-11-21(16)33-5/h8-11,13-14H,6-7,12,15H2,1-5H3,(H2,25,27,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human leutinizing releasing hormone receptor |
Bioorg Med Chem Lett 15: 2265-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.030
BindingDB Entry DOI: 10.7270/Q2GB23K1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149840
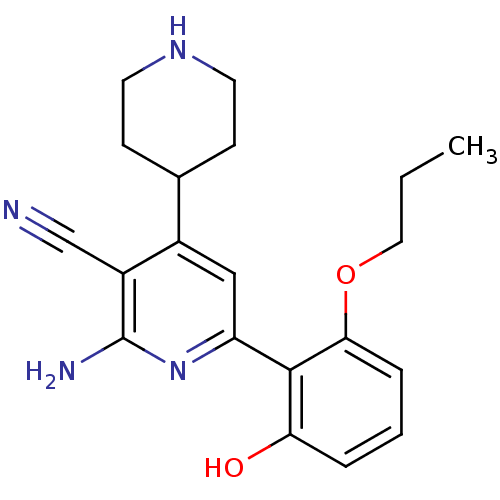
(2-Amino-6-(2-hydroxy-6-propoxy-phenyl)-1'',2'',3''...)Show InChI InChI=1S/C20H24N4O2/c1-2-10-26-18-5-3-4-17(25)19(18)16-11-14(13-6-8-23-9-7-13)15(12-21)20(22)24-16/h3-5,11,13,23,25H,2,6-10H2,1H3,(H2,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50149806
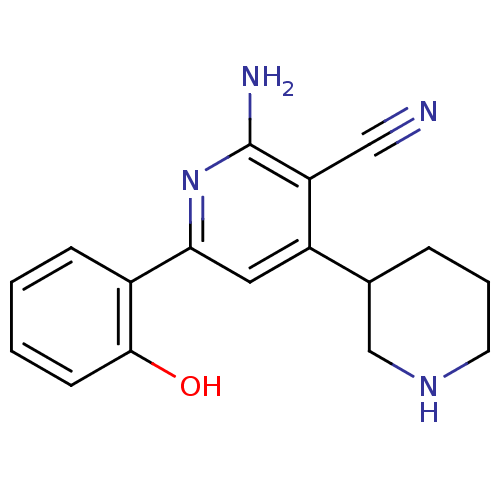
(2''-Amino-6''-(2-hydroxy-phenyl)-1,2,3,4,5,6-hexah...)Show InChI InChI=1S/C17H18N4O/c18-9-14-13(11-4-3-7-20-10-11)8-15(21-17(14)19)12-5-1-2-6-16(12)22/h1-2,5-6,8,11,20,22H,3-4,7,10H2,(H2,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of I-kappa-B-kinase alpha |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149806

(2''-Amino-6''-(2-hydroxy-phenyl)-1,2,3,4,5,6-hexah...)Show InChI InChI=1S/C17H18N4O/c18-9-14-13(11-4-3-7-20-10-11)8-15(21-17(14)19)12-5-1-2-6-16(12)22/h1-2,5-6,8,11,20,22H,3-4,7,10H2,(H2,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human I-kappa-B-kinase beta |
Bioorg Med Chem Lett 14: 4013-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.040
BindingDB Entry DOI: 10.7270/Q2PC31VC |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149834
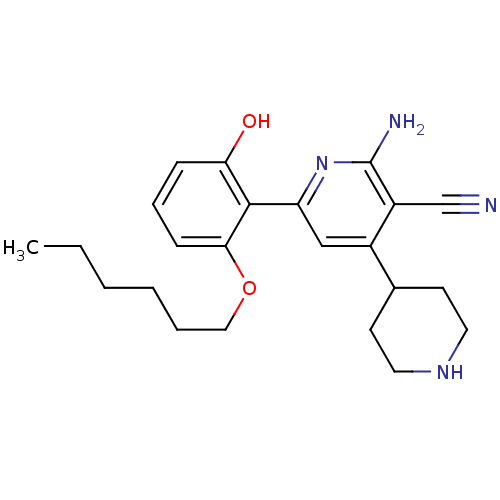
(2-Amino-6-(2-hexyloxy-6-hydroxy-phenyl)-1'',2'',3'...)Show SMILES CCCCCCOc1cccc(O)c1-c1cc(C2CCNCC2)c(C#N)c(N)n1 Show InChI InChI=1S/C23H30N4O2/c1-2-3-4-5-13-29-21-8-6-7-20(28)22(21)19-14-17(16-9-11-26-12-10-16)18(15-24)23(25)27-19/h6-8,14,16,26,28H,2-5,9-13H2,1H3,(H2,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149806

(2''-Amino-6''-(2-hydroxy-phenyl)-1,2,3,4,5,6-hexah...)Show InChI InChI=1S/C17H18N4O/c18-9-14-13(11-4-3-7-20-10-11)8-15(21-17(14)19)12-5-1-2-6-16(12)22/h1-2,5-6,8,11,20,22H,3-4,7,10H2,(H2,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50149835
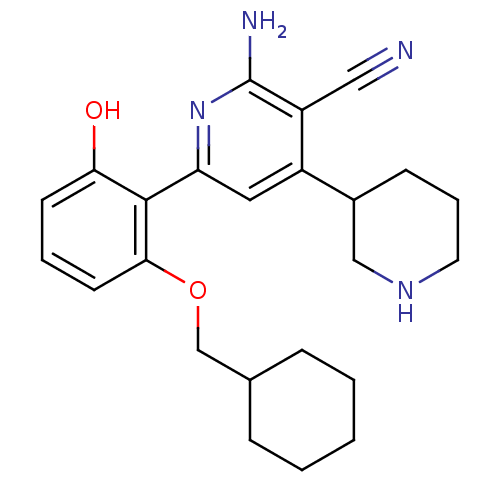
(2''-Amino-6''-(2-cyclohexylmethoxy-6-hydroxy-pheny...)Show SMILES Nc1nc(cc(C2CCCNC2)c1C#N)-c1c(O)cccc1OCC1CCCCC1 Show InChI InChI=1S/C24H30N4O2/c25-13-19-18(17-8-5-11-27-14-17)12-20(28-24(19)26)23-21(29)9-4-10-22(23)30-15-16-6-2-1-3-7-16/h4,9-10,12,16-17,27,29H,1-3,5-8,11,14-15H2,(H2,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 619-0216
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human I-kappa-B-kinase beta (IKK beta) |
Bioorg Med Chem Lett 14: 4019-22 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.041
BindingDB Entry DOI: 10.7270/Q2JM293M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data