| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Metabotropic glutamate receptor 1 |
|---|
| Ligand | BDBM50224402 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_450777 (CHEMBL899862) |
|---|
| Ki | 2.4±n/a nM |
|---|
| Citation |  Wu, WL; Burnett, DA; Domalski, M; Greenlee, WJ; Li, C; Bertorelli, R; Fredduzzi, S; Lozza, G; Veltri, A; Reggiani, A Discovery of orally efficacious tetracyclic metabotropic glutamate receptor 1 (mGluR1) antagonists for the treatment of chronic pain. J Med Chem50:5550-3 (2007) [PubMed] Article Wu, WL; Burnett, DA; Domalski, M; Greenlee, WJ; Li, C; Bertorelli, R; Fredduzzi, S; Lozza, G; Veltri, A; Reggiani, A Discovery of orally efficacious tetracyclic metabotropic glutamate receptor 1 (mGluR1) antagonists for the treatment of chronic pain. J Med Chem50:5550-3 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Metabotropic glutamate receptor 1 |
|---|
| Name: | Metabotropic glutamate receptor 1 |
|---|
| Synonyms: | GRM1_RAT | Gprc1a | Grm1 | Metabotropic Glutamate 1a | Metabotropic glutamate receptor | Metabotropic glutamate receptor 1 | Mglur1 | metabotropic glutamate 1 | metabotropic glutamate 1/5-D | metabotropic glutamate 1/DA | metabotropic glutamate receptor 1 isoform alpha precursor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 133240.47 |
|---|
| Organism: | RAT |
|---|
| Description: | metabotropic glutamate 1/2 0 RAT::P23385 |
|---|
| Residue: | 1199 |
|---|
| Sequence: | MVRLLLIFFPMIFLEMSILPRMPDRKVLLAGASSQRSVARMDGDVIIGALFSVHHQPPAE
KVPERKCGEIREQYGIQRVEAMFHTLDKINADPVLLPNITLGSEIRDSCWHSSVALEQSI
EFIRDSLISIRDEKDGLNRCLPDGQTLPPGRTKKPIAGVIGPGSSSVAIQVQNLLQLFDI
PQIAYSATSIDLSDKTLYKYFLRVVPSDTLQARAMLDIVKRYNWTYVSAVHTEGNYGESG
MDAFKELAAQEGLCIAHSDKIYSNAGEKSFDRLLRKLRERLPKARVVVCFCEGMTVRGLL
SAMRRLGVVGEFSLIGSDGWADRDEVIEGYEVEANGGITIKLQSPEVRSFDDYFLKLRLD
TNTRNPWFPEFWQHRFQCRLPGHLLENPNFKKVCTGNESLEENYVQDSKMGFVINAIYAM
AHGLQNMHHALCPGHVGLCDAMKPIDGRKLLDFLIKSSFVGVSGEEVWFDEKGDAPGRYD
IMNLQYTEANRYDYVHVGTWHEGVLNIDDYKIQMNKSGMVRSVCSEPCLKGQIKVIRKGE
VSCCWICTACKENEFVQDEFTCRACDLGWWPNAELTGCEPIPVRYLEWSDIESIIAIAFS
CLGILVTLFVTLIFVLYRDTPVVKSSSRELCYIILAGIFLGYVCPFTLIAKPTTTSCYLQ
RLLVGLSSAMCYSALVTKTNRIARILAGSKKKICTRKPRFMSAWAQVIIASILISVQLTL
VVTLIIMEPPMPILSYPSIKEVYLICNTSNLGVVAPVGYNGLLIMSCTYYAFKTRNVPAN
FNEAKYIAFTMYTTCIIWLAFVPIYFGSNYKIITTCFAVSLSVTVALGCMFTPKMYIIIA
KPERNVRSAFTTSDVVRMHVGDGKLPCRSNTFLNIFRRKKPGAGNANSNGKSVSWSEPGG
RQAPKGQHVWQRLSVHVKTNETACNQTAVIKPLTKSYQGSGKSLTFSDASTKTLYNVEEE
DNTPSAHFSPPSSPSMVVHRRGPPVATTPPLPPHLTAEETPLFLADSVIPKGLPPPLPQQ
QPQQPPPQQPPQQPKSLMDQLQGVVTNFGSGIPDFHAVLAGPGTPGNSLRSLYPPPPPPQ
HLQMLPLHLSTFQEESISPPGEDIDDDSERFKLLQEFVYEREGNTEEDELEEEEDLPTAS
KLTPEDSPALTPPSPFRDSVASGSSVPSSPVSESVLCTPPNVTYASVILRDYKQSSSTL
|
|
|
|---|
| BDBM50224402 |
|---|
| n/a |
|---|
| Name | BDBM50224402 |
|---|
| Synonyms: | 8-(4-chlorophenyl)-1h-pyrrolo[2'',3'':4',5']pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-7(8H)-one | CHEMBL238077 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H9ClN4OS |
|---|
| Mol. Mass. | 352.798 |
|---|
| SMILES | Clc1ccc(cc1)-n1cnc2c(sc3ncc4cc[nH]c4c23)c1=O |
|---|
| Structure | 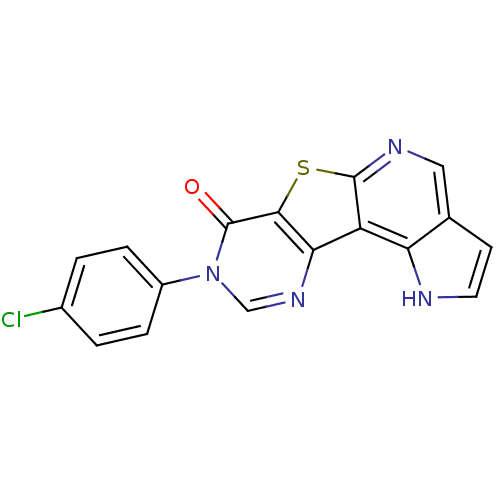
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wu, WL; Burnett, DA; Domalski, M; Greenlee, WJ; Li, C; Bertorelli, R; Fredduzzi, S; Lozza, G; Veltri, A; Reggiani, A Discovery of orally efficacious tetracyclic metabotropic glutamate receptor 1 (mGluR1) antagonists for the treatment of chronic pain. J Med Chem50:5550-3 (2007) [PubMed] Article
Wu, WL; Burnett, DA; Domalski, M; Greenlee, WJ; Li, C; Bertorelli, R; Fredduzzi, S; Lozza, G; Veltri, A; Reggiani, A Discovery of orally efficacious tetracyclic metabotropic glutamate receptor 1 (mGluR1) antagonists for the treatment of chronic pain. J Med Chem50:5550-3 (2007) [PubMed] Article