

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306442 ((6aS,13bS)-11-chloro-7-methyl-4-phenyl-6,6a,7,8,9,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306314 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224406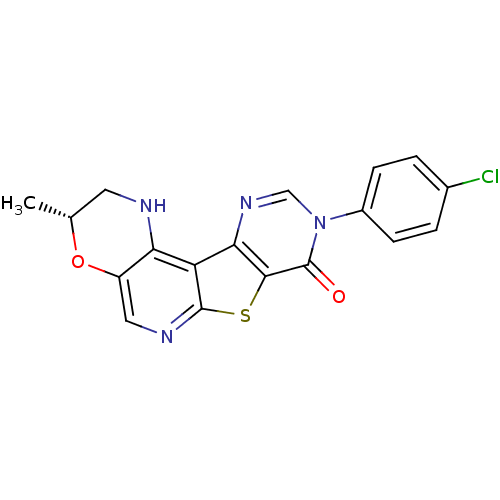 (9-(4-chlorophenyl)-2,3-dihydro-3(R)-methyl-1H-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306440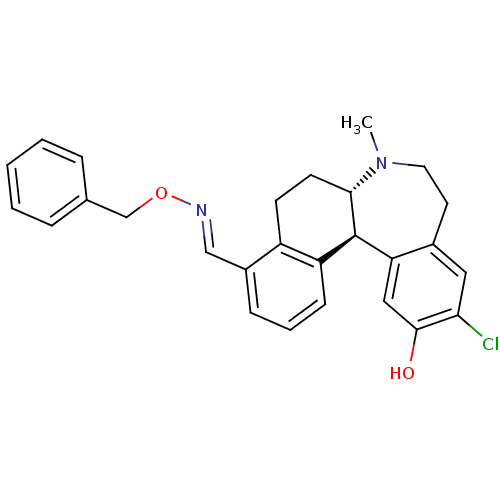 (11-chloro-12-hydroxy-7-methyl-6,6a,7,8,9,13b-hexah...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345941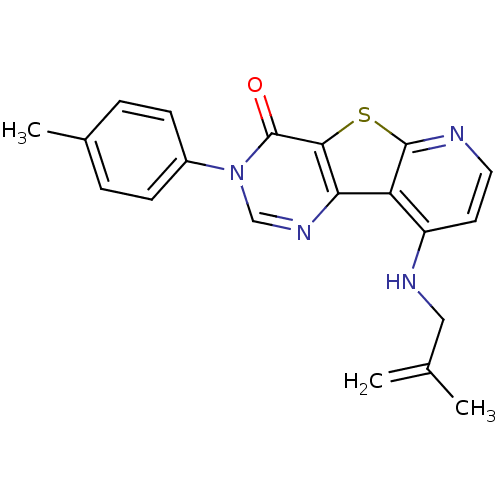 (9-(2-Methyl-allylamino)-3-p-tolyl-3H-pyrido[3',2':...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224407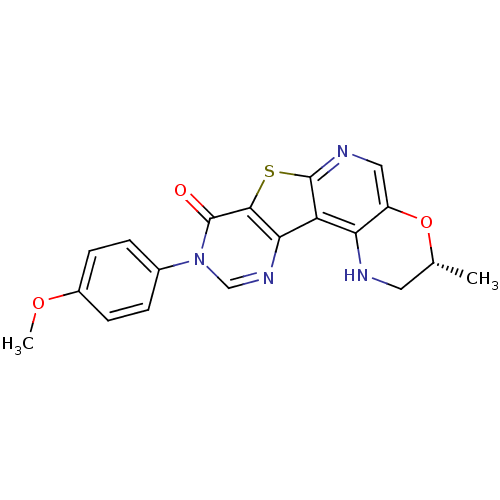 (2,3-dihydro-9-(4-methoxyphenyl)-3(R)-methyl-1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190709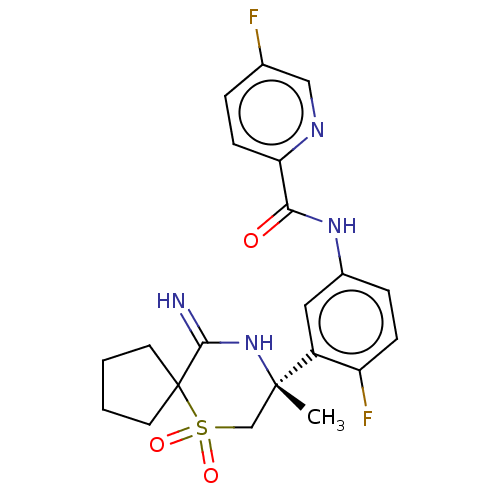 (US9181236, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306452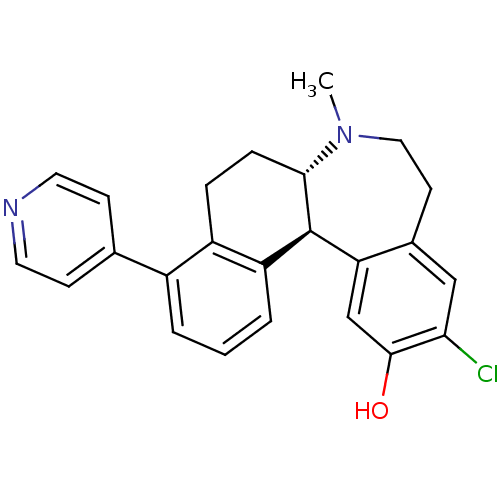 ((6aS,13bS)-11-chloro-7-methyl-4-(pyridin-4-yl)-6,6...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190708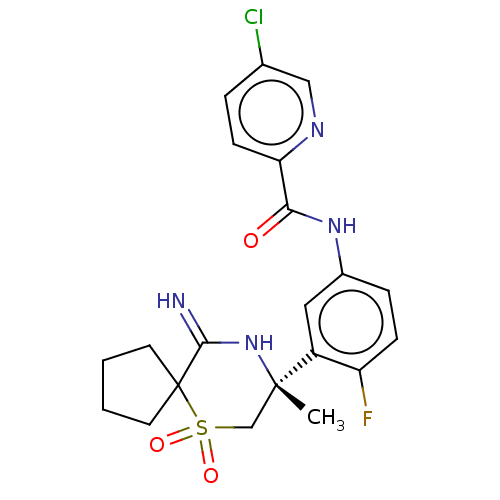 (US9181236, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM249123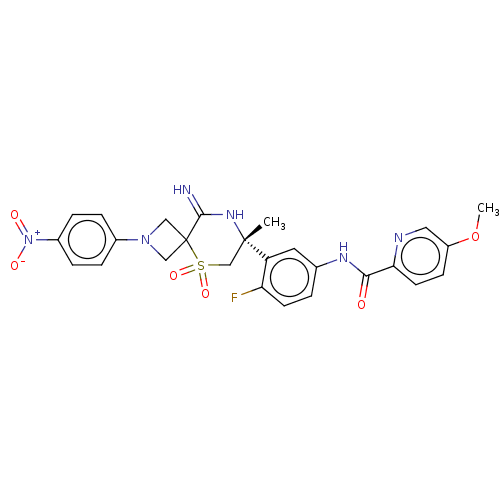 (US9453034, 42) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.320 | -55.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEVN... | US Patent US9453034 (2016) BindingDB Entry DOI: 10.7270/Q27P8XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224391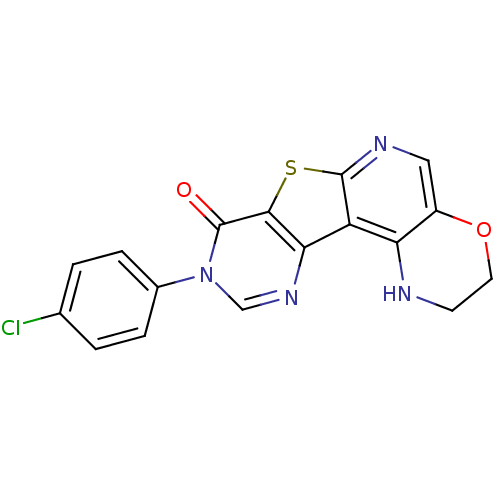 (9-(4-chlorophenyl)-2,3-dihydro-1H-pyrimido[4'',5''...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50364723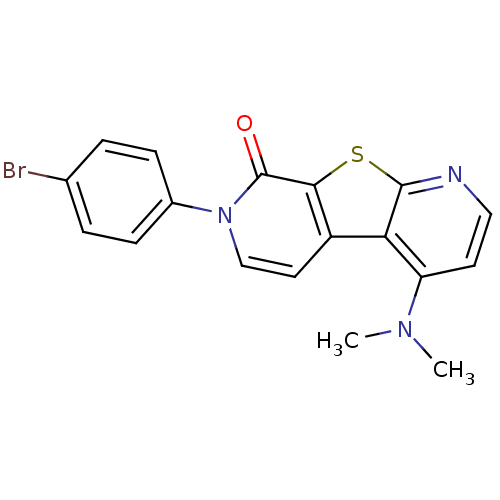 (CHEMBL1951662) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat metabotropic glutamate receptor 1 | Bioorg Med Chem Lett 22: 1575-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.131 BindingDB Entry DOI: 10.7270/Q2F19063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190697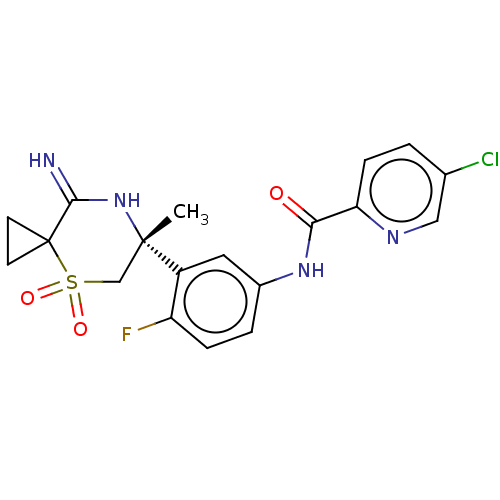 (US9181236, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM249091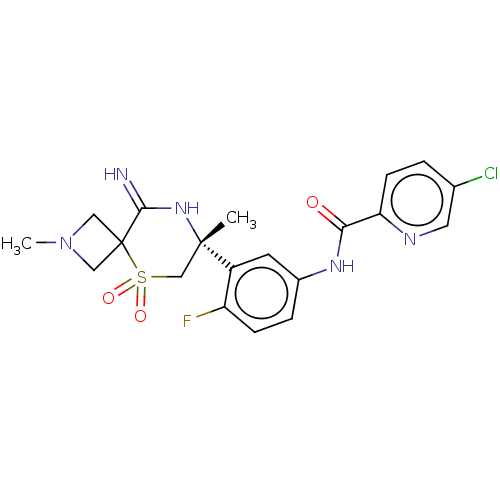 (US9453034, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEVN... | US Patent US9453034 (2016) BindingDB Entry DOI: 10.7270/Q27P8XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345942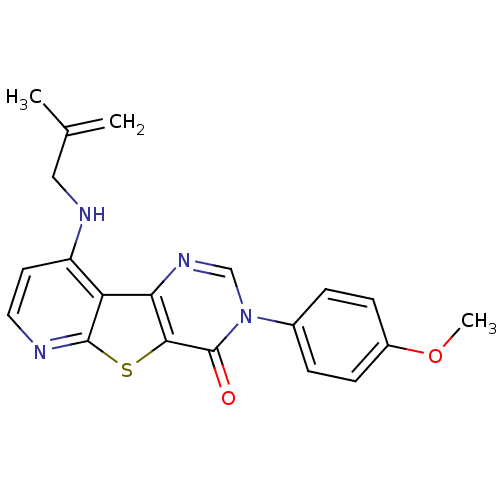 (3-(4-Methoxy-phenyl)-9-(2-methyl-allylamino)-3H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306443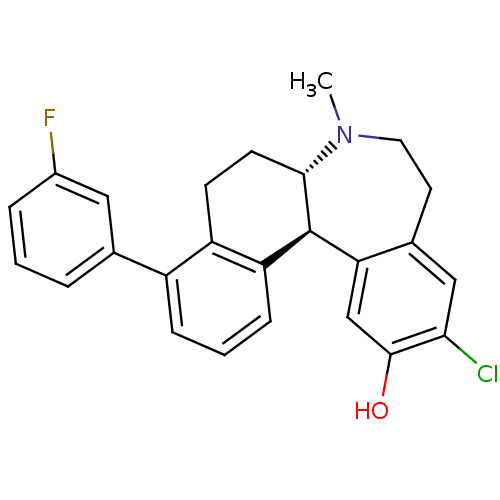 ((6aS,13bS)-11-chloro-4-(3-fluorophenyl)-7-methyl-6...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258901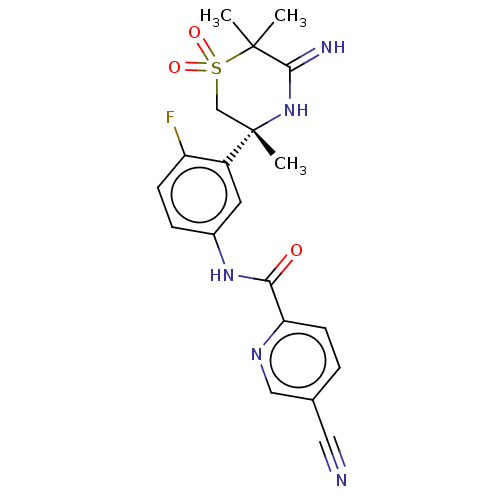 (US9499502, 9b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.5 | <-54.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306315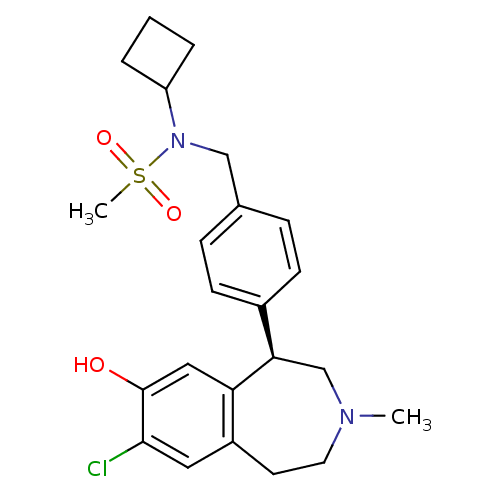 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM249100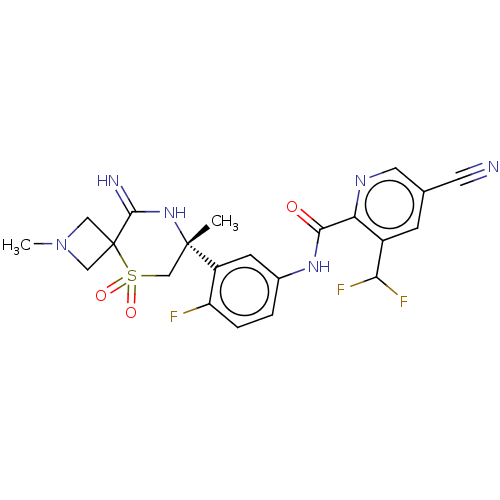 (US9453034, 12b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEVN... | US Patent US9453034 (2016) BindingDB Entry DOI: 10.7270/Q27P8XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306322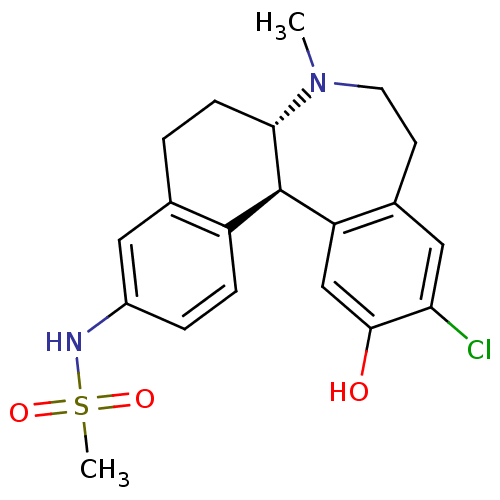 (CHEMBL597909 | N-((6aS,13bR)-11-chloro-12-hydroxy-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258899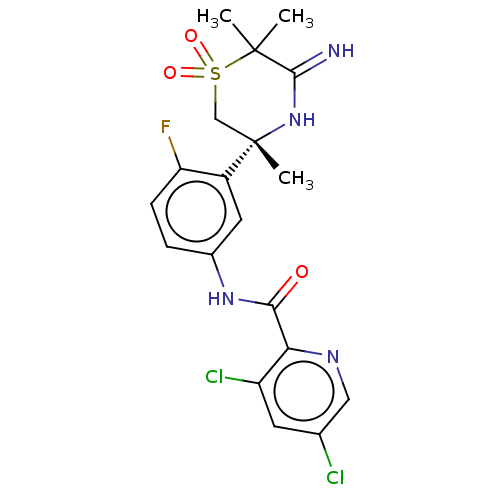 (US9499502, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.5 | <-54.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224392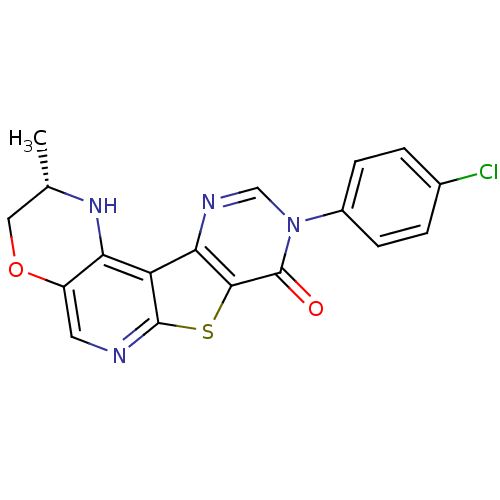 (9-(4-chlorophenyl)-2,3-dihydro-2(S)-methyl-1H-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224395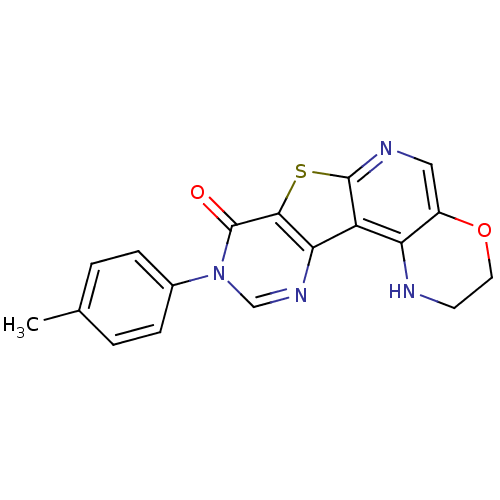 (2,3-dihydro-9-(4-methylphenyl)-1H-pyrimido[4'',5''...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306316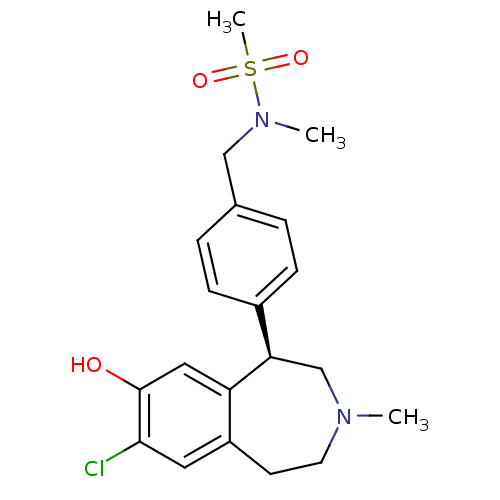 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190700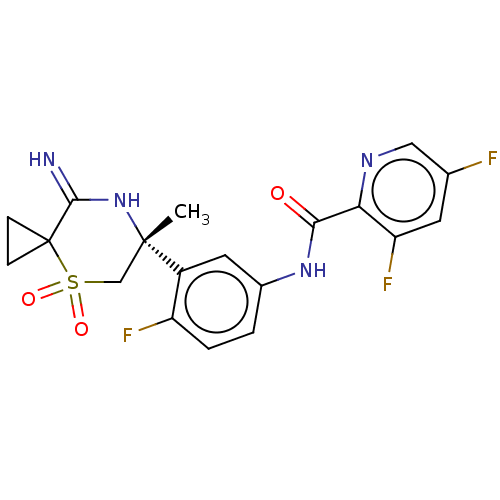 (US9181236, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224400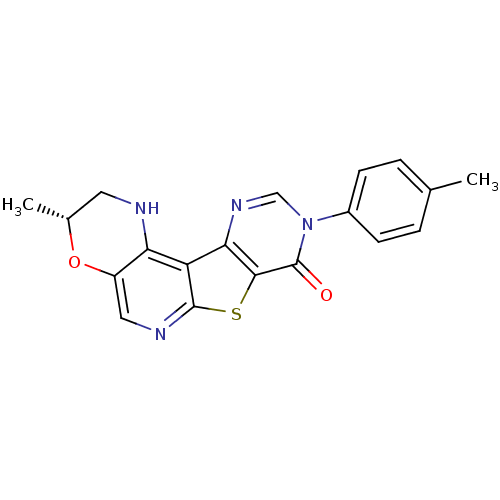 (2,3-dihydro-3(R)-methyl-9-(4-methylphenyl)-1H-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306444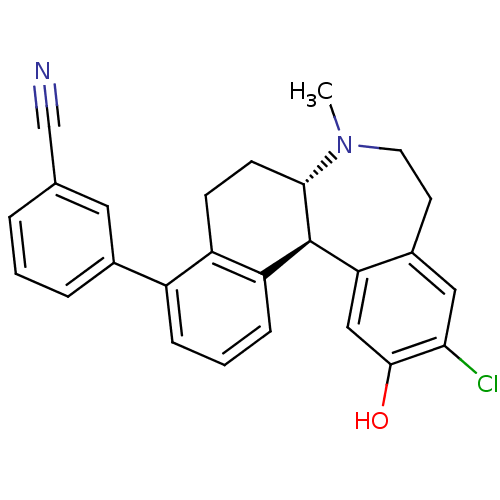 (3-((6aS,13bS)-11-chloro-12-hydroxy-7-methyl-6,6a,7...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306317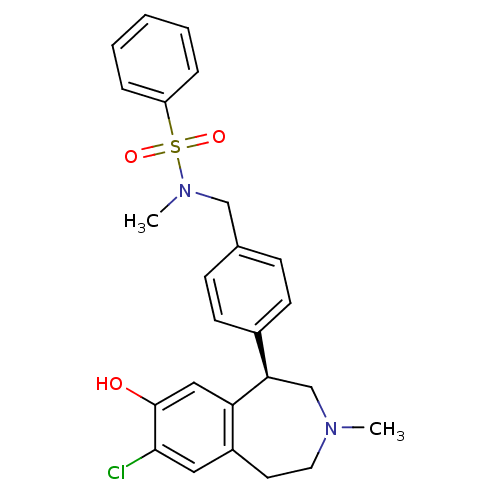 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM249121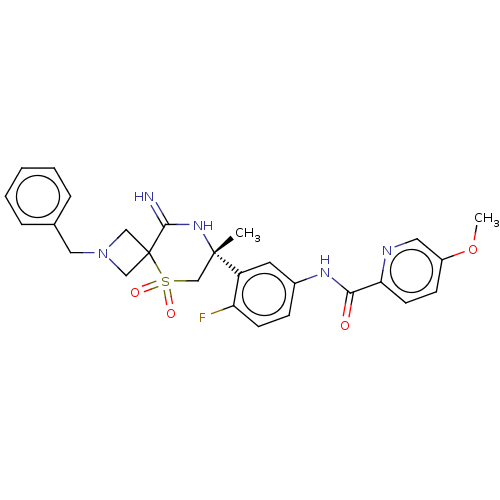 (US9453034, 40) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEVN... | US Patent US9453034 (2016) BindingDB Entry DOI: 10.7270/Q27P8XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306445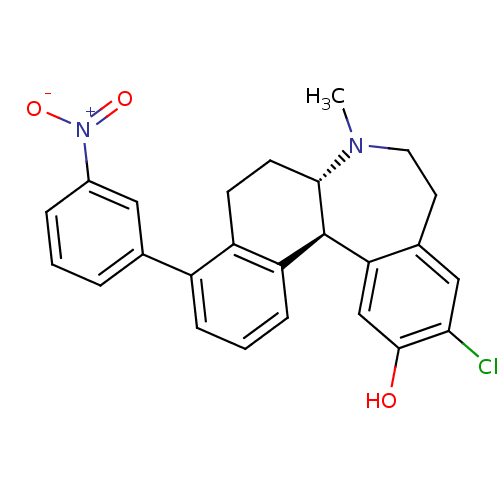 ((6aS,13bS)-11-chloro-7-methyl-4-(3-nitrophenyl)-6,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258902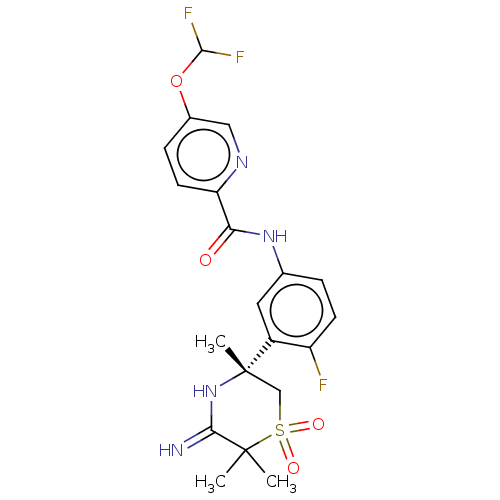 (US9499502, 9c) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345947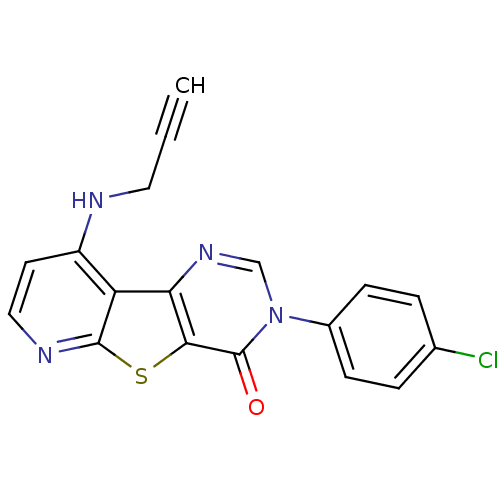 (3-(4-Chloro-phenyl)-9-prop-2-ynylamino-3H-pyrido[3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258897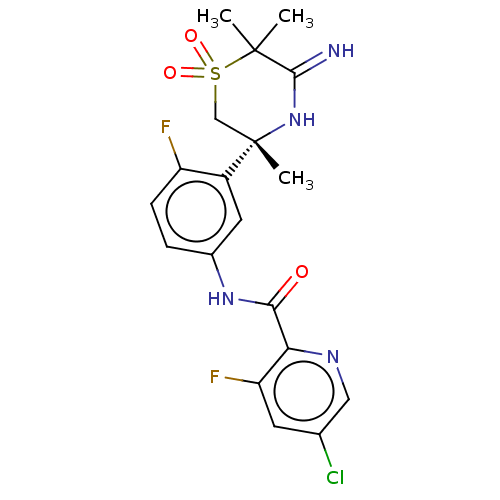 (US9499502, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306453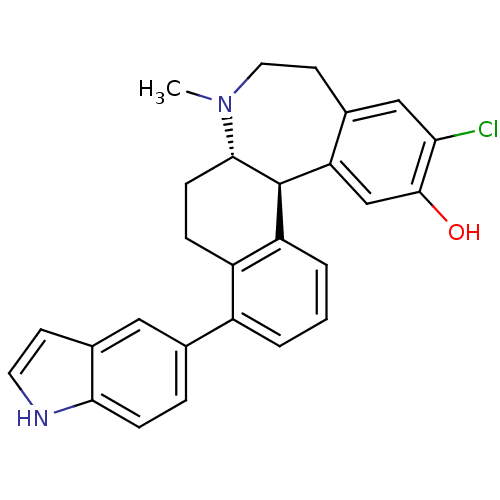 ((6aS,13bS)-11-chloro-4-(1H-indol-5-yl)-7-methyl-6,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258909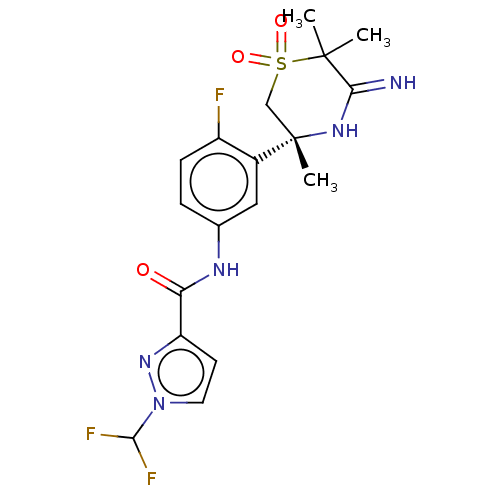 (US9499502, 9j) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190711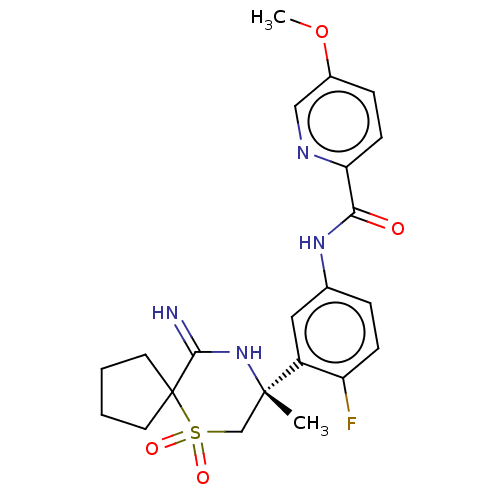 (US9181236, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -53.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306435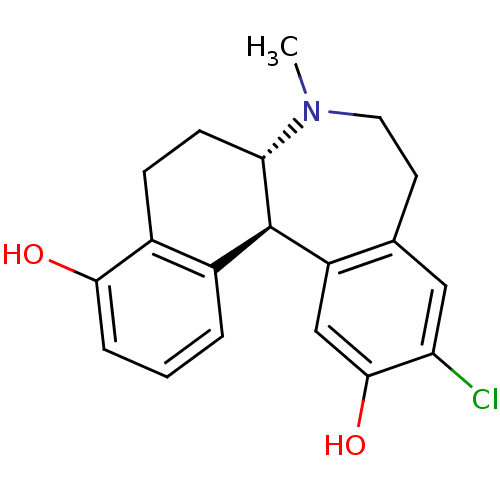 ((6aS,13bS)-11-chloro-7-methyl-6,6a,7,8,9,13b-hexah...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306450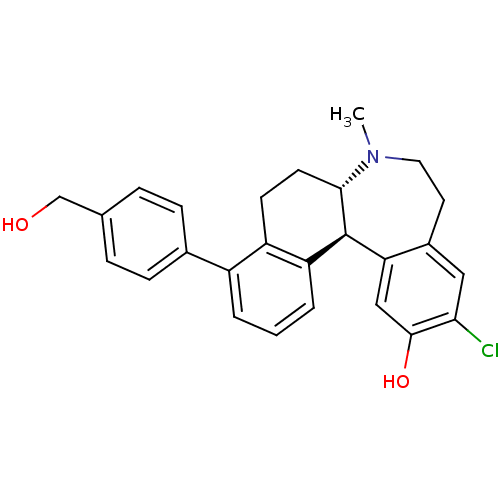 ((6aS,13bS)-11-chloro-4-(4-(hydroxymethyl)phenyl)-7...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306449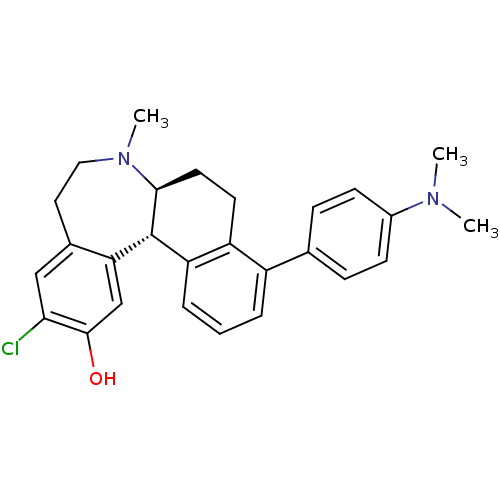 ((6aS,13bS)-11-chloro-4-(4-(dimethylamino)phenyl)-7...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306448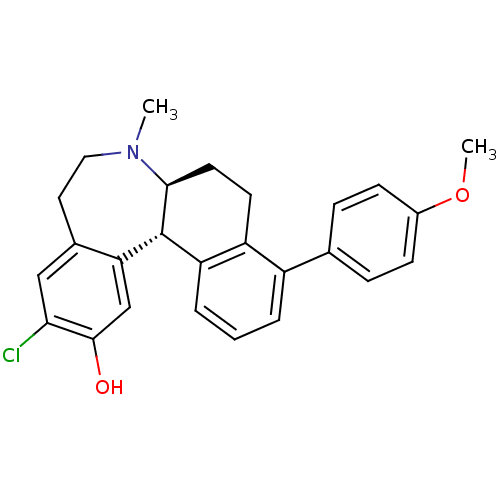 ((6aS,13bS)-11-chloro-4-(4-methoxyphenyl)-7-methyl-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258904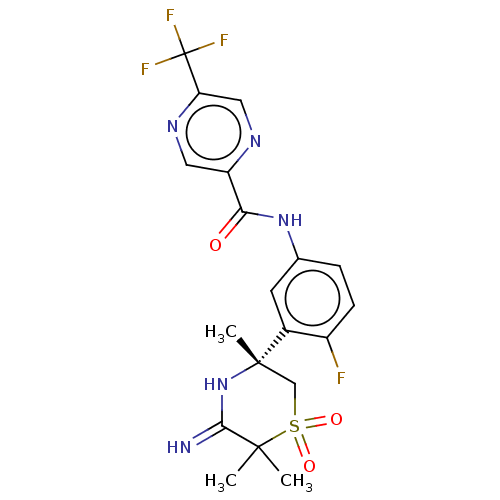 (US9499502, 9e) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -53.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258896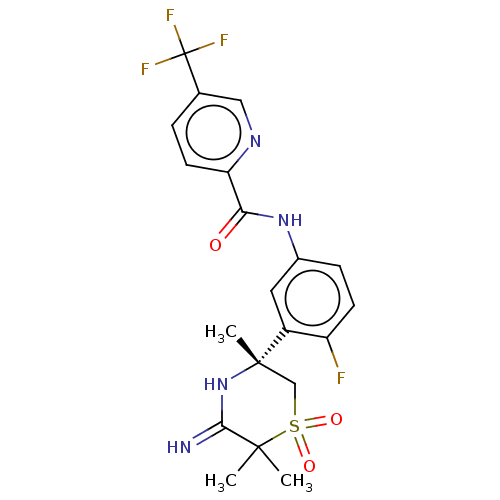 (US9499502, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -53.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM258893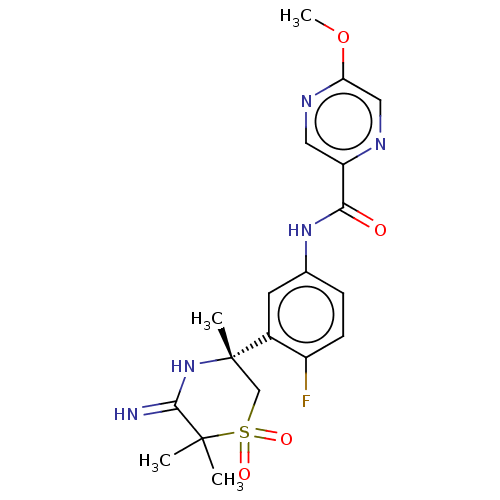 (US9499502, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -53.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay can be used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. This assay mo... | US Patent US9499502 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345934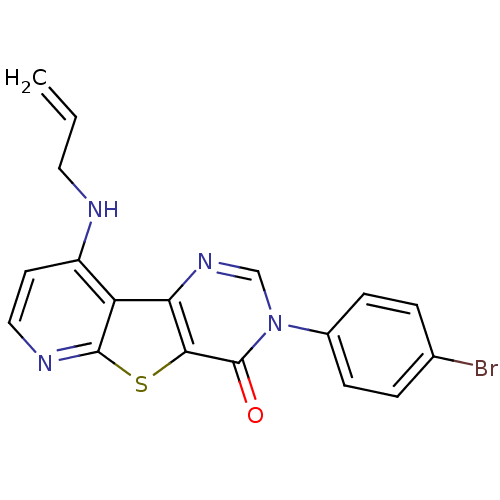 (9-Allylamino-3-(4-bromo-phenyl)-3H-pyrido[3',2':4,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50345950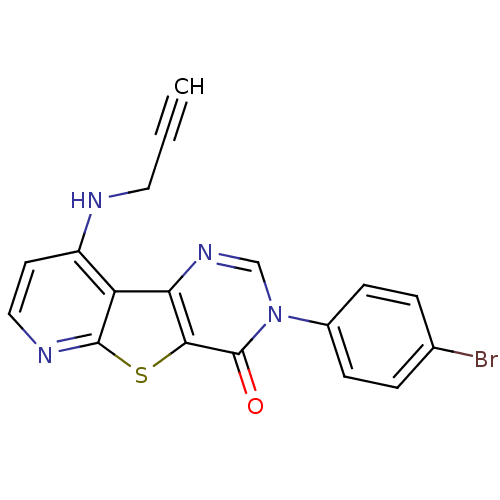 (3-(4-Bromo-phenyl)-9-prop-2-ynylamino-3H-pyrido[3'...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 | Bioorg Med Chem Lett 19: 3199-203 (2009) Article DOI: 10.1016/j.bmcl.2009.04.104 BindingDB Entry DOI: 10.7270/Q27081SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306325 (1-((6aS,13bR)-11-chloro-12-hydroxy-7-methyl-6,6a,7...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306323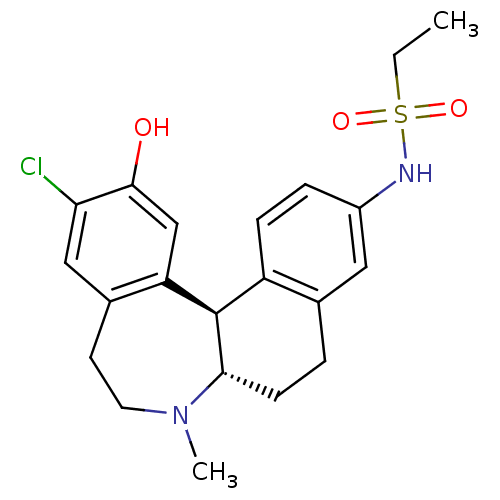 (CHEMBL600986 | N-((6aS,13bR)-11-chloro-12-hydroxy-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306319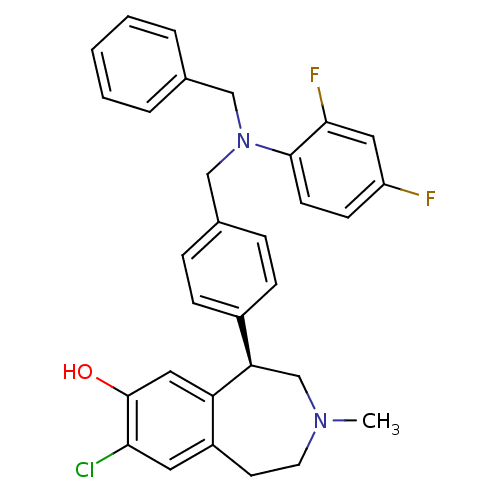 ((R)-5-(4-((benzyl(2,4-difluorophenyl)amino)methyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM249099 (US9453034, 12a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | -52.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEVN... | US Patent US9453034 (2016) BindingDB Entry DOI: 10.7270/Q27P8XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM190714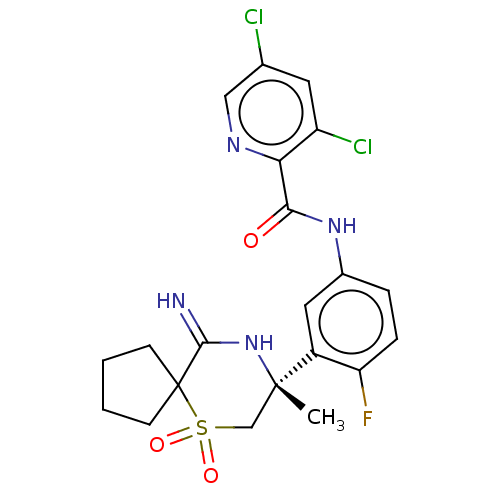 (US9181236, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -52.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISE... | US Patent US9181236 (2015) BindingDB Entry DOI: 10.7270/Q2NC5ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3677 total ) | Next | Last >> |