| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50262988 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_490564 (CHEMBL981170) |
|---|
| IC50 | 0.12±n/a nM |
|---|
| Citation |  Leonetti, F; Catto, M; Nicolotti, O; Pisani, L; Cappa, A; Stefanachi, A; Carotti, A Homo- and hetero-bivalent edrophonium-like ammonium salts as highly potent, dual binding site AChE inhibitors. Bioorg Med Chem16:7450-6 (2008) [PubMed] Article Leonetti, F; Catto, M; Nicolotti, O; Pisani, L; Cappa, A; Stefanachi, A; Carotti, A Homo- and hetero-bivalent edrophonium-like ammonium salts as highly potent, dual binding site AChE inhibitors. Bioorg Med Chem16:7450-6 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_BOVIN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase precursor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 67659.62 |
|---|
| Organism: | Bos taurus (bovine) |
|---|
| Description: | n/a |
|---|
| Residue: | 613 |
|---|
| Sequence: | MRPPWCPLHTPSLTPPLLLLLFLIGGGAEAEGPEDPELLVMVRGGRLRGLRLMAPRGPVS
AFLGIPFAEPPVGPRRFLPPEPKRPWPGVLNATAFQSVCYQYVDTLYPGFEGTEMWNPNR
ELSEDCLYLNVWTPYPRPSSPTPVLVWIYGGGFYSGASSLDVYDGRFLTQAEGTVLVSMN
YRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASVG
MHLLSPPSRGLFHRAVLQSGAPNGPWATVGVGEARRRATLLARLVGCPPGGAGGNDTELV
ACLRARPAQDLVDHEWRVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVGV
VKDEGSYFLVYGAPGFSKDNESLISRAQFLAGVRVGVPQASDLAAEAVVLHYTDWLHPED
PARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYIFEHRASTLSWPLWMGVPHGYE
IEFIFGLPLEPSLNYTIEERTFAQRLMRYWANFARTGDPNDPRDPKAPQWPPYTAGAQQY
VSLNLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKNQ
FDHYSKQDRCSDL
|
|
|
|---|
| BDBM50262988 |
|---|
| n/a |
|---|
| Name | BDBM50262988 |
|---|
| Synonyms: | CHEMBL1200541 | N-(2-chlorobenzyl)-2-(2-(2-((2-chlorobenzyl)diethylammonio)ethylamino)-2-oxoacetamido)-N,N-diethylethanaminium | ambenonium |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H42Cl2N4O2 |
|---|
| Mol. Mass. | 537.564 |
|---|
| SMILES | CC[N+](CC)(CCNC(=O)C(=O)NCC[N+](CC)(CC)Cc1ccccc1Cl)Cc1ccccc1Cl |
|---|
| Structure | 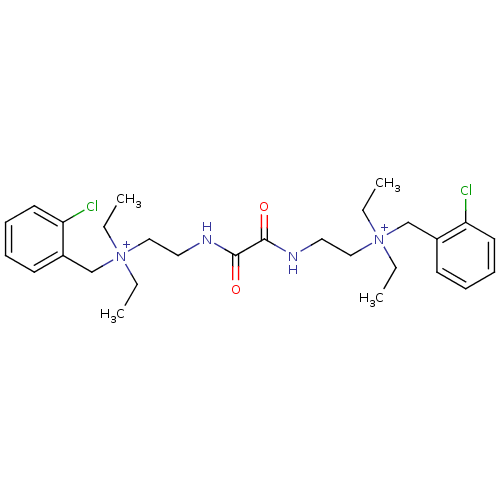
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Leonetti, F; Catto, M; Nicolotti, O; Pisani, L; Cappa, A; Stefanachi, A; Carotti, A Homo- and hetero-bivalent edrophonium-like ammonium salts as highly potent, dual binding site AChE inhibitors. Bioorg Med Chem16:7450-6 (2008) [PubMed] Article
Leonetti, F; Catto, M; Nicolotti, O; Pisani, L; Cappa, A; Stefanachi, A; Carotti, A Homo- and hetero-bivalent edrophonium-like ammonium salts as highly potent, dual binding site AChE inhibitors. Bioorg Med Chem16:7450-6 (2008) [PubMed] Article