| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Leukotriene A-4 hydrolase |
|---|
| Ligand | BDBM50272060 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_510221 (CHEMBL1004837) |
|---|
| IC50 | 3600±n/a nM |
|---|
| Citation |  Enomoto, H; Morikawa, Y; Miyake, Y; Tsuji, F; Mizuchi, M; Suhara, H; Fujimura, K; Horiuchi, M; Ban, M Synthesis and biological evaluation of N-mercaptoacylproline and N-mercaptoacylthiazolidine-4-carboxylic acid derivatives as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett18:4529-32 (2008) [PubMed] Article Enomoto, H; Morikawa, Y; Miyake, Y; Tsuji, F; Mizuchi, M; Suhara, H; Fujimura, K; Horiuchi, M; Ban, M Synthesis and biological evaluation of N-mercaptoacylproline and N-mercaptoacylthiazolidine-4-carboxylic acid derivatives as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett18:4529-32 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Leukotriene A-4 hydrolase |
|---|
| Name: | Leukotriene A-4 hydrolase |
|---|
| Synonyms: | LKHA4_CAVPO | LTA4H |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 68972.78 |
|---|
| Organism: | Cavia porcellus |
|---|
| Description: | ChEMBL_544515 |
|---|
| Residue: | 611 |
|---|
| Sequence: | MPEVVDTCSLASPATVCRTKHLHLRCSVDFTRRALTGVAALTIQSQEDNLRSLILDTKDL
TIEKVVINGQEVKYALGEKQSYKGSPMEISLPIALSKNQEVVIEISFETSPKSSALQWLT
PEQTSGKEHPYLFSQCQAIHCRAFLPCQDTPSVKLTYTAEVSVPKELVALMSAIRDGEAP
DPADPSRKIYKFSQKVPIPCYLIALVVGALESRKIGPRTLVWSEKEQVDKSAYEFSETES
MLKIAEDLGGPYVWGQYDRLVLPPSFSYGGMENPCLTFVTPTLLAGDKSLSNVIAHEISH
TWTGNLVTNKTWDHFWLNEGHTVYLERHICGRLFGEKFRHFHALGGWGELQNTVKTLGET
QAFTKLVVDLTDTDPDVAYSSVPYEKGFALLFHLEQLLGGPEVFLGFLKAYVEKFSYKSI
TTDDWKNFLFSHFKDKVDILNQVDWDAWLYSPGLPPIKPNYDMTLTNACIALSQRWITAK
EKDLNTFSATDLKDLSSHQVNEFLAQVLQRAPLPLGHVKRMQEVYNCNAINNSEIRFRWL
RLCIQSKWEEAIPLALKMATEQGRMKFTRPLFKDLAAFDKSHDQAIQTYHAHKASMHPVT
AMLVGKDLKVE
|
|
|
|---|
| BDBM50272060 |
|---|
| n/a |
|---|
| Name | BDBM50272060 |
|---|
| Synonyms: | (2S,5S)-5-(benzylthio)-1-((S)-3-mercapto-2-methylpropanoyl)pyrrolidine-2-carboxylic acid | CHEMBL498798 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H21NO3S2 |
|---|
| Mol. Mass. | 339.473 |
|---|
| SMILES | C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCc1ccccc1 |r| |
|---|
| Structure | 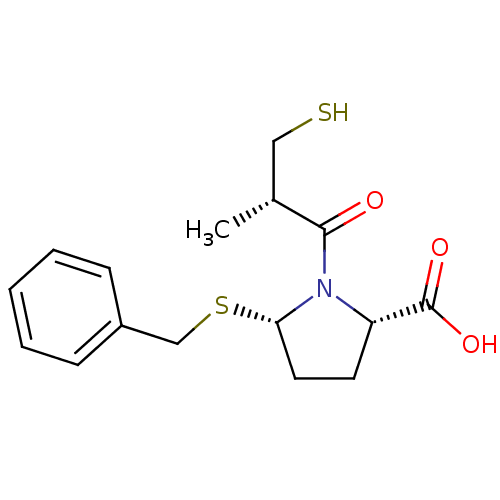
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Enomoto, H; Morikawa, Y; Miyake, Y; Tsuji, F; Mizuchi, M; Suhara, H; Fujimura, K; Horiuchi, M; Ban, M Synthesis and biological evaluation of N-mercaptoacylproline and N-mercaptoacylthiazolidine-4-carboxylic acid derivatives as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett18:4529-32 (2008) [PubMed] Article
Enomoto, H; Morikawa, Y; Miyake, Y; Tsuji, F; Mizuchi, M; Suhara, H; Fujimura, K; Horiuchi, M; Ban, M Synthesis and biological evaluation of N-mercaptoacylproline and N-mercaptoacylthiazolidine-4-carboxylic acid derivatives as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett18:4529-32 (2008) [PubMed] Article