 Found 160 hits with Last Name = 'morikawa' and Initial = 'y'
Found 160 hits with Last Name = 'morikawa' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241817
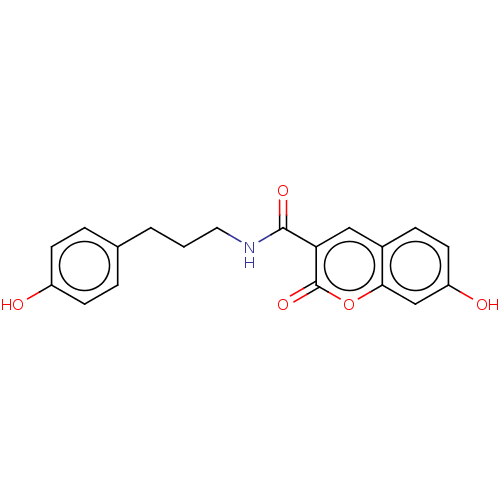
(CHEMBL4081954)Show InChI InChI=1S/C19H17NO5/c21-14-6-3-12(4-7-14)2-1-9-20-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,21-22H,1-2,9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human AKR1B10 in presence of geraniol as substrate by Lineweaver-Burk plot method |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272062
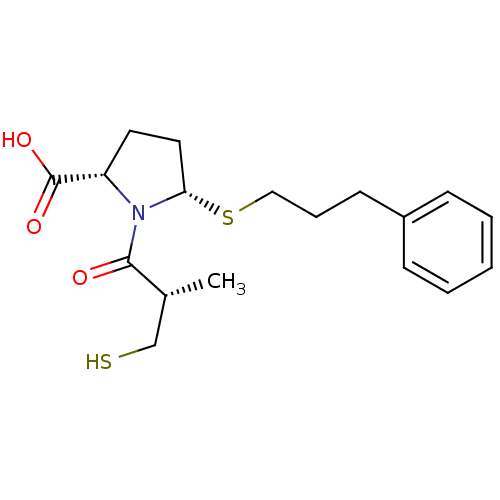
((2S,5S)-1-((S)-3-mercapto-2-methylpropanoyl)-5-(3-...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCCCc1ccccc1 |r| Show InChI InChI=1S/C18H25NO3S2/c1-13(12-23)17(20)19-15(18(21)22)9-10-16(19)24-11-5-8-14-6-3-2-4-7-14/h2-4,6-7,13,15-16,23H,5,8-12H2,1H3,(H,21,22)/t13-,15+,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272061
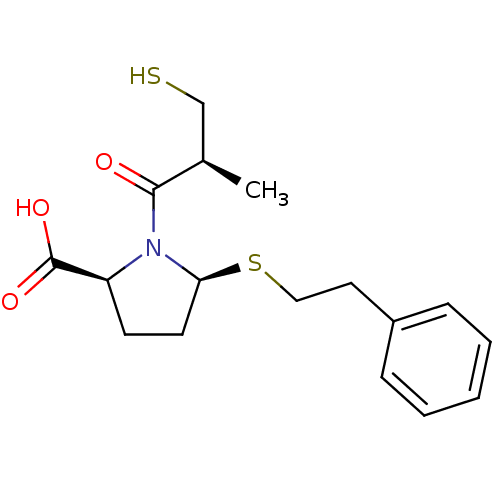
((2S,5S)-1-((S)-3-mercapto-2-methylpropanoyl)-5-(ph...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCCc1ccccc1 |r| Show InChI InChI=1S/C17H23NO3S2/c1-12(11-22)16(19)18-14(17(20)21)7-8-15(18)23-10-9-13-5-3-2-4-6-13/h2-6,12,14-15,22H,7-11H2,1H3,(H,20,21)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272145

((2S,5S)-1-((S)-3-Mercapto-2-methyl-propionyl)-5-(3...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCc1cccc(C)c1 |r| Show InChI InChI=1S/C17H23NO3S2/c1-11-4-3-5-13(8-11)10-23-15-7-6-14(17(20)21)18(15)16(19)12(2)9-22/h3-5,8,12,14-15,22H,6-7,9-10H2,1-2H3,(H,20,21)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272199
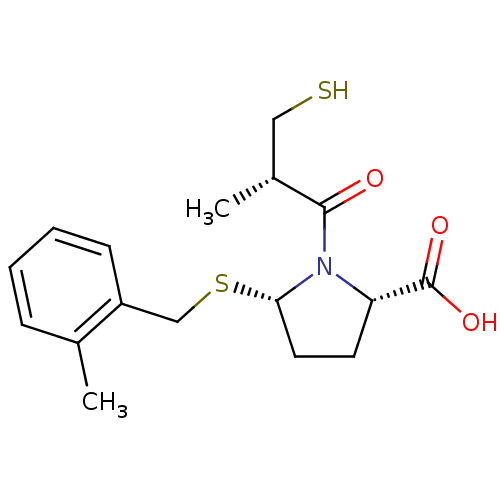
((2S,5S)-1-((S)-3-Mercapto-2-methyl-propionyl)-5-(2...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCc1ccccc1C |r| Show InChI InChI=1S/C17H23NO3S2/c1-11-5-3-4-6-13(11)10-23-15-8-7-14(17(20)21)18(15)16(19)12(2)9-22/h3-6,12,14-15,22H,7-10H2,1-2H3,(H,20,21)/t12-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272060
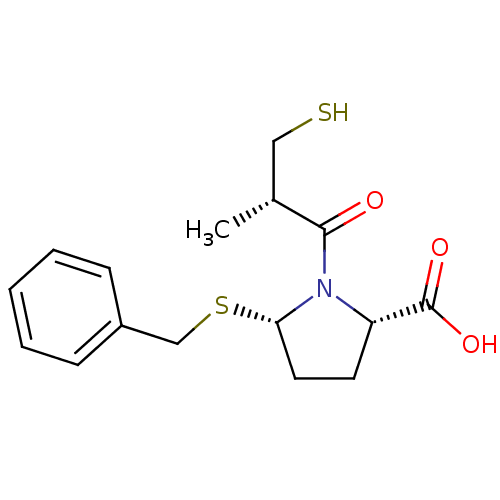
((2S,5S)-5-(benzylthio)-1-((S)-3-mercapto-2-methylp...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCc1ccccc1 |r| Show InChI InChI=1S/C16H21NO3S2/c1-11(9-21)15(18)17-13(16(19)20)7-8-14(17)22-10-12-5-3-2-4-6-12/h2-6,11,13-14,21H,7-10H2,1H3,(H,19,20)/t11-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272099
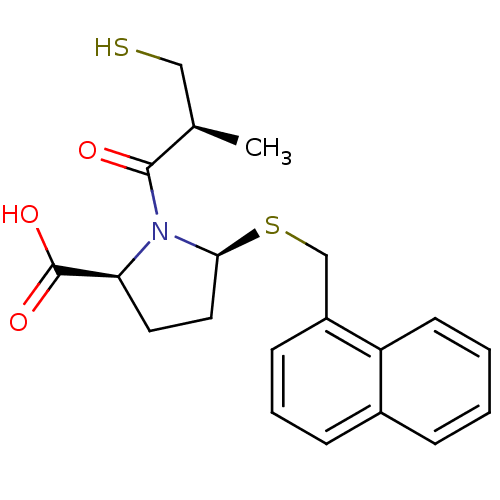
((2S,5S)-1-((S)-3-mercapto-2-methylpropanoyl)-5-(na...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCc1cccc2ccccc12 |r| Show InChI InChI=1S/C20H23NO3S2/c1-13(11-25)19(22)21-17(20(23)24)9-10-18(21)26-12-15-7-4-6-14-5-2-3-8-16(14)15/h2-8,13,17-18,25H,9-12H2,1H3,(H,23,24)/t13-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272203

((2S,5R)-5-(4-isopropylbenzylthio)-1-((S)-3-mercapt...)Show SMILES CC(C)c1ccc(CS[C@@H]2CC[C@H](N2C(=O)[C@H](C)CS)C(O)=O)cc1 |r| Show InChI InChI=1S/C19H27NO3S2/c1-12(2)15-6-4-14(5-7-15)11-25-17-9-8-16(19(22)23)20(17)18(21)13(3)10-24/h4-7,12-13,16-17,24H,8-11H2,1-3H3,(H,22,23)/t13-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241828
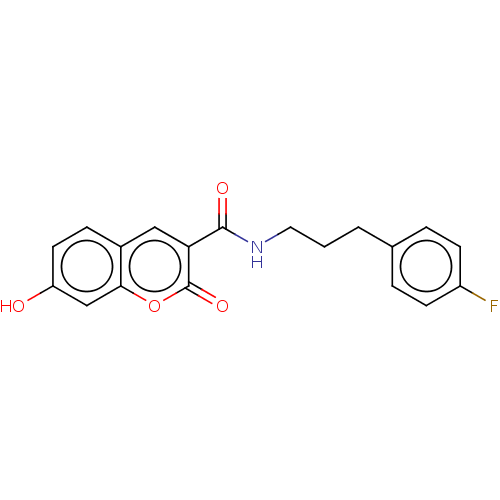
(CHEMBL4089817)Show InChI InChI=1S/C19H16FNO4/c20-14-6-3-12(4-7-14)2-1-9-21-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,22H,1-2,9H2,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272200
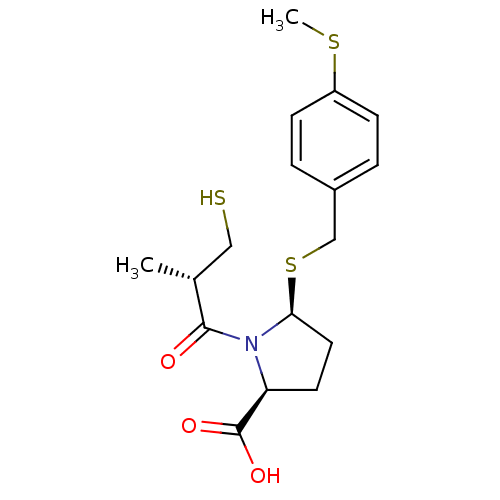
((2S,5S)-5-(4-(methylthio)benzylthio)-1-((S)-3-merc...)Show SMILES CSc1ccc(CS[C@H]2CC[C@H](N2C(=O)[C@H](C)CS)C(O)=O)cc1 |r| Show InChI InChI=1S/C17H23NO3S3/c1-11(9-22)16(19)18-14(17(20)21)7-8-15(18)24-10-12-3-5-13(23-2)6-4-12/h3-6,11,14-15,22H,7-10H2,1-2H3,(H,20,21)/t11-,14+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241817

(CHEMBL4081954)Show InChI InChI=1S/C19H17NO5/c21-14-6-3-12(4-7-14)2-1-9-20-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,21-22H,1-2,9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272141
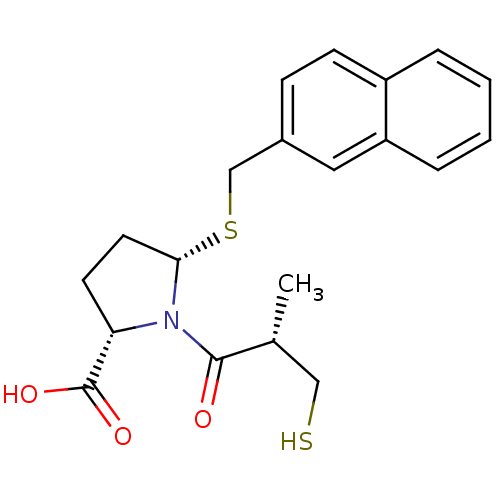
((2S,5S)-1-((S)-3-mercapto-2-methylpropanoyl)-5-(na...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H23NO3S2/c1-13(11-25)19(22)21-17(20(23)24)8-9-18(21)26-12-14-6-7-15-4-2-3-5-16(15)10-14/h2-7,10,13,17-18,25H,8-9,11-12H2,1H3,(H,23,24)/t13-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50442489

(CHEMBL2440417)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H20N2O4/c1-29-20-11-8-18(9-12-20)26-24-21(13-17-7-10-19(27)14-22(17)30-24)23(28)25-15-16-5-3-2-4-6-16/h2-14,27H,15H2,1H3,(H,25,28)/b26-24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1B10 (unknown origin) |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241818
(CHEMBL4060049)Show InChI InChI=1S/C19H16FNO4/c20-14-5-1-3-12(9-14)4-2-8-21-18(23)16-10-13-6-7-15(22)11-17(13)25-19(16)24/h1,3,5-7,9-11,22H,2,4,8H2,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241816

(CHEMBL4098452)Show InChI InChI=1S/C19H17NO5/c21-14-8-7-13-10-15(19(24)25-17(13)11-14)18(23)20-9-3-5-12-4-1-2-6-16(12)22/h1-2,4,6-8,10-11,21-22H,3,5,9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241822

(CHEMBL4062779)Show InChI InChI=1S/C20H19NO4/c1-13-4-6-14(7-5-13)3-2-10-21-19(23)17-11-15-8-9-16(22)12-18(15)25-20(17)24/h4-9,11-12,22H,2-3,10H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241826
(CHEMBL4089816)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)NCCCc1cccc(O)c1 Show InChI InChI=1S/C26H24N2O5/c1-32-22-11-8-19(9-12-22)28-26-23(15-18-7-10-21(30)16-24(18)33-26)25(31)27-13-3-5-17-4-2-6-20(29)14-17/h2,4,6-12,14-16,29-30H,3,5,13H2,1H3,(H,27,31)/b28-26- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241821

(CHEMBL4068704)Show SMILES Oc1ccc2cc(C(=O)NCCCc3cc(F)cc(F)c3)c(=O)oc2c1 Show InChI InChI=1S/C19H15F2NO4/c20-13-6-11(7-14(21)9-13)2-1-5-22-18(24)16-8-12-3-4-15(23)10-17(12)26-19(16)25/h3-4,6-10,23H,1-2,5H2,(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241827

(CHEMBL4071421)Show InChI InChI=1S/C19H17NO5/c21-14-5-1-3-12(9-14)4-2-8-20-18(23)16-10-13-6-7-15(22)11-17(13)25-19(16)24/h1,3,5-7,9-11,21-22H,2,4,8H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272142
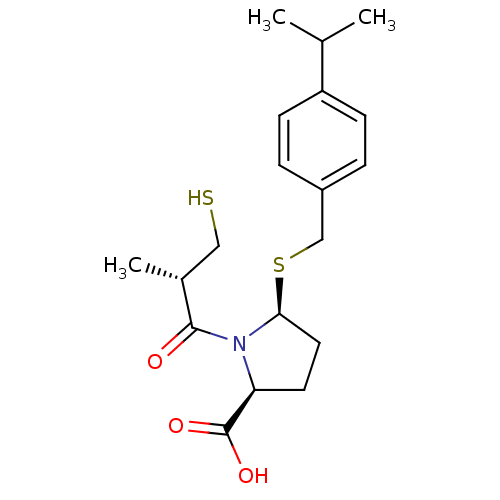
((2S,5S)-5-(4-isopropylbenzylthio)-1-((S)-3-mercapt...)Show SMILES CC(C)c1ccc(CS[C@H]2CC[C@H](N2C(=O)[C@H](C)CS)C(O)=O)cc1 |r| Show InChI InChI=1S/C19H27NO3S2/c1-12(2)15-6-4-14(5-7-15)11-25-17-9-8-16(19(22)23)20(17)18(21)13(3)10-24/h4-7,12-13,16-17,24H,8-11H2,1-3H3,(H,22,23)/t13-,16+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM16314
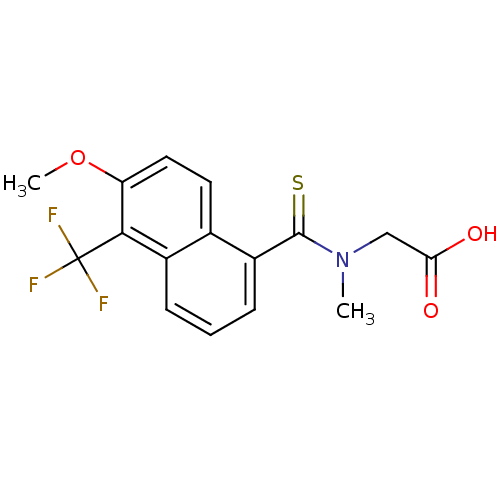
(2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...)Show InChI InChI=1S/C16H14F3NO3S/c1-20(8-13(21)22)15(24)11-5-3-4-10-9(11)6-7-12(23-2)14(10)16(17,18)19/h3-7H,8H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1B1 assessed as decrease in glyceraldehyde reduction |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241829
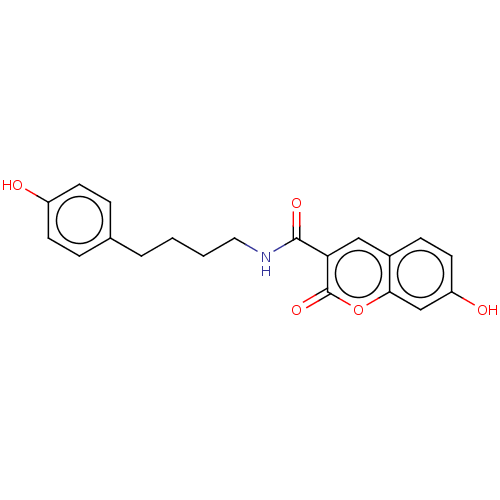
(CHEMBL4071420)Show InChI InChI=1S/C20H19NO5/c22-15-7-4-13(5-8-15)3-1-2-10-21-19(24)17-11-14-6-9-16(23)12-18(14)26-20(17)25/h4-9,11-12,22-23H,1-3,10H2,(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241823

(CHEMBL4084456)Show InChI InChI=1S/C20H19NO5/c1-25-16-8-4-13(5-9-16)3-2-10-21-19(23)17-11-14-6-7-15(22)12-18(14)26-20(17)24/h4-9,11-12,22H,2-3,10H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241826

(CHEMBL4089816)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)NCCCc1cccc(O)c1 Show InChI InChI=1S/C26H24N2O5/c1-32-22-11-8-19(9-12-22)28-26-23(15-18-7-10-21(30)16-24(18)33-26)25(31)27-13-3-5-17-4-2-6-20(29)14-17/h2,4,6-12,14-16,29-30H,3,5,13H2,1H3,(H,27,31)/b28-26- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272144
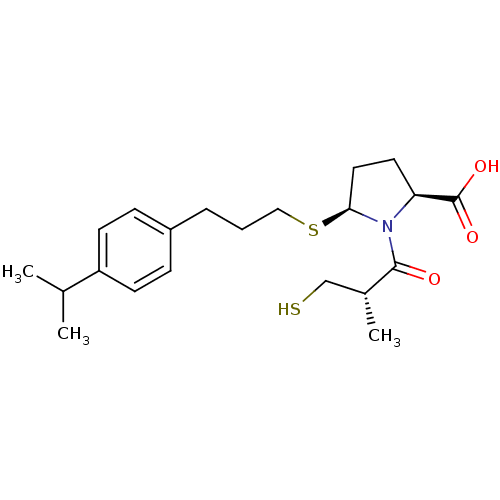
((2S,5S)-5-(3-(4-isopropylphenyl)propylthio)-1-((S)...)Show SMILES CC(C)c1ccc(CCCS[C@H]2CC[C@H](N2C(=O)[C@H](C)CS)C(O)=O)cc1 |r| Show InChI InChI=1S/C21H31NO3S2/c1-14(2)17-8-6-16(7-9-17)5-4-12-27-19-11-10-18(21(24)25)22(19)20(23)15(3)13-26/h6-9,14-15,18-19,26H,4-5,10-13H2,1-3H3,(H,24,25)/t15-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50266136
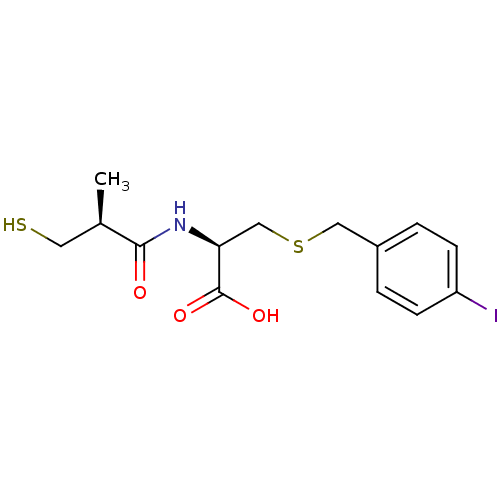
((R)-3-(4-iodobenzylthio)-2-((S)-3-mercapto-2-methy...)Show SMILES C[C@H](CS)C(=O)N[C@@H](CSCc1ccc(I)cc1)C(O)=O |r| Show InChI InChI=1S/C14H18INO3S2/c1-9(6-20)13(17)16-12(14(18)19)8-21-7-10-2-4-11(15)5-3-10/h2-5,9,12,20H,6-8H2,1H3,(H,16,17)(H,18,19)/t9-,12+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase in guinea pig lung assessed as inhibition of LTB4 production |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50272143
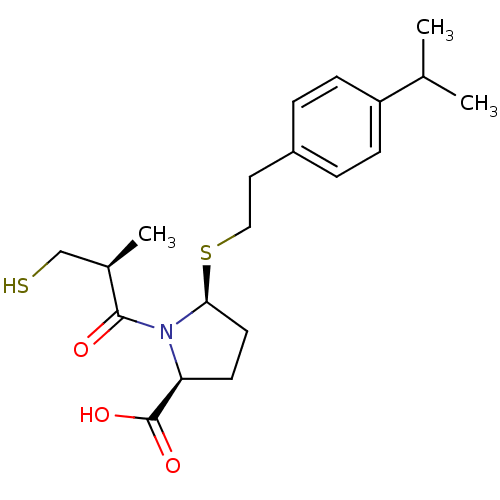
((2S,5S)-5-(4-isopropylphenethylthio)-1-((S)-3-merc...)Show SMILES CC(C)c1ccc(CCS[C@H]2CC[C@H](N2C(=O)[C@H](C)CS)C(O)=O)cc1 |r| Show InChI InChI=1S/C20H29NO3S2/c1-13(2)16-6-4-15(5-7-16)10-11-26-18-9-8-17(20(23)24)21(18)19(22)14(3)12-25/h4-7,13-14,17-18,25H,8-12H2,1-3H3,(H,23,24)/t14-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241830
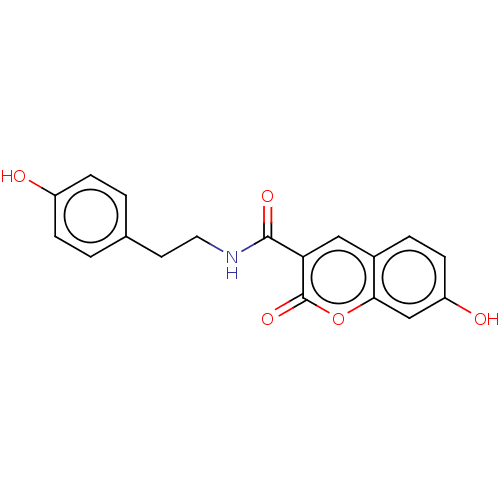
(CHEMBL3318218)Show InChI InChI=1S/C18H15NO5/c20-13-4-1-11(2-5-13)7-8-19-17(22)15-9-12-3-6-14(21)10-16(12)24-18(15)23/h1-6,9-10,20-21H,7-8H2,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50266170
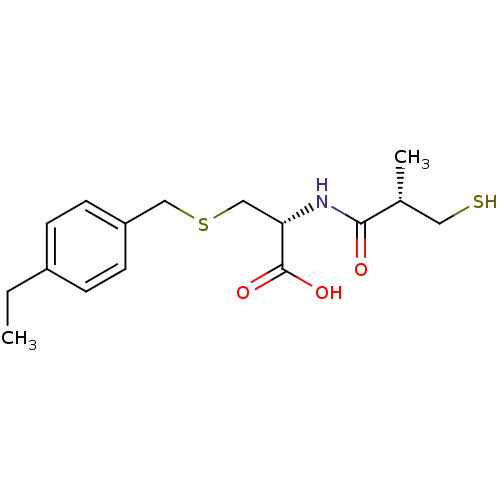
((R)-3-(4-ethylbenzylthio)-2-((S)-3-mercapto-2-meth...)Show SMILES CCc1ccc(CSC[C@H](NC(=O)[C@H](C)CS)C(O)=O)cc1 |r| Show InChI InChI=1S/C16H23NO3S2/c1-3-12-4-6-13(7-5-12)9-22-10-14(16(19)20)17-15(18)11(2)8-21/h4-7,11,14,21H,3,8-10H2,1-2H3,(H,17,18)(H,19,20)/t11-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin-converting enzyme (unknown origin) in whole blood by HPLC |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50442489

(CHEMBL2440417)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H20N2O4/c1-29-20-11-8-18(9-12-20)26-24-21(13-17-7-10-19(27)14-22(17)30-24)23(28)25-15-16-5-3-2-4-6-16/h2-14,27H,15H2,1H3,(H,25,28)/b26-24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1B1 (unknown origin) |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50266173
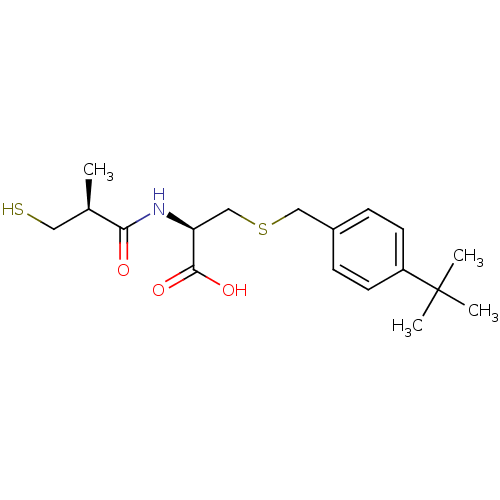
((R)-3-(4-tert-butylbenzylthio)-2-((S)-3-mercapto-2...)Show SMILES C[C@H](CS)C(=O)N[C@@H](CSCc1ccc(cc1)C(C)(C)C)C(O)=O |r| Show InChI InChI=1S/C18H27NO3S2/c1-12(9-23)16(20)19-15(17(21)22)11-24-10-13-5-7-14(8-6-13)18(2,3)4/h5-8,12,15,23H,9-11H2,1-4H3,(H,19,20)(H,21,22)/t12-,15+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase in guinea pig lung assessed as inhibition of LTB4 production |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50394657

(CHEMBL270067)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16-,17+,18+,19-,20+,21+,23+,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human intestinal N-terminal 6-His-tagged AKR1B10 expressed in Escherichia coli BL21 (DE3) pLysS cells using pyridine-3-alde... |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50272201
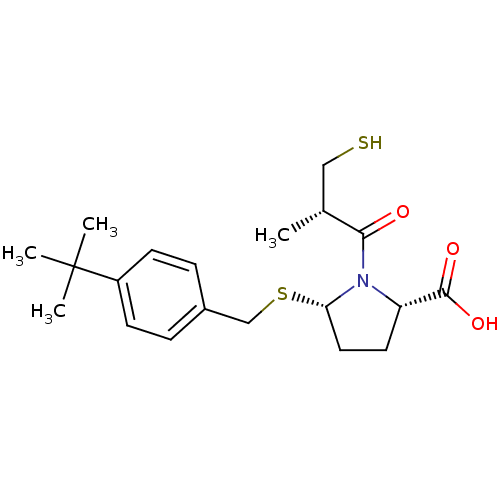
((2S,5S)-5-(4-tert-butylbenzylthio)-1-((S)-3-mercap...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCc1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C20H29NO3S2/c1-13(11-25)18(22)21-16(19(23)24)9-10-17(21)26-12-14-5-7-15(8-6-14)20(2,3)4/h5-8,13,16-17,25H,9-12H2,1-4H3,(H,23,24)/t13-,16+,17+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig lung leukotriene A4 hydrolase |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50272202
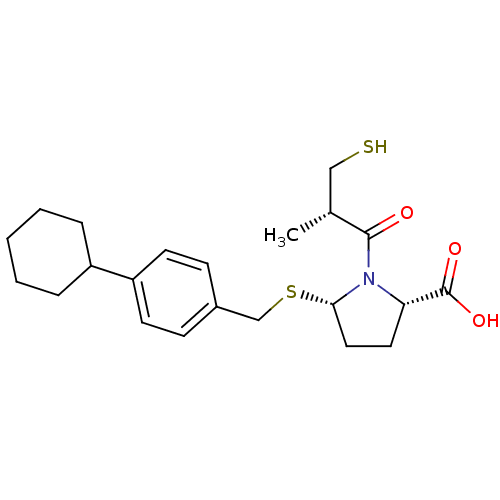
((2S,5S)-5-(4-cyclohexylbenzylthio)-1-((S)-3-mercap...)Show SMILES C[C@H](CS)C(=O)N1[C@H](CC[C@H]1C(O)=O)SCc1ccc(cc1)C1CCCCC1 |r| Show InChI InChI=1S/C22H31NO3S2/c1-15(13-27)21(24)23-19(22(25)26)11-12-20(23)28-14-16-7-9-18(10-8-16)17-5-3-2-4-6-17/h7-10,15,17,19-20,27H,2-6,11-14H2,1H3,(H,25,26)/t15-,19+,20+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig lung leukotriene A4 hydrolase |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50266075
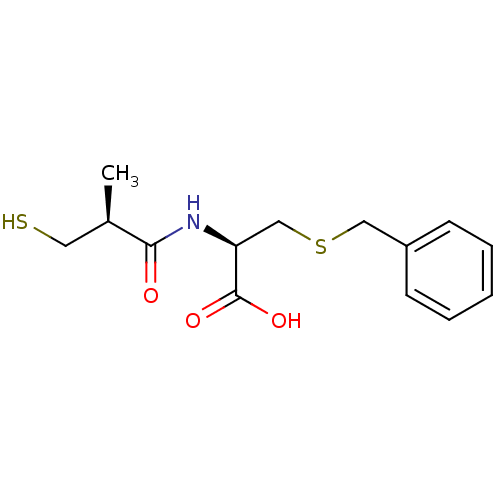
((R)-3-(benzylthio)-2-((S)-3-mercapto-2-methylpropa...)Show SMILES C[C@H](CS)C(=O)N[C@@H](CSCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C14H19NO3S2/c1-10(7-19)13(16)15-12(14(17)18)9-20-8-11-5-3-2-4-6-11/h2-6,10,12,19H,7-9H2,1H3,(H,15,16)(H,17,18)/t10-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin-converting enzyme (unknown origin) in whole blood by HPLC |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50266244
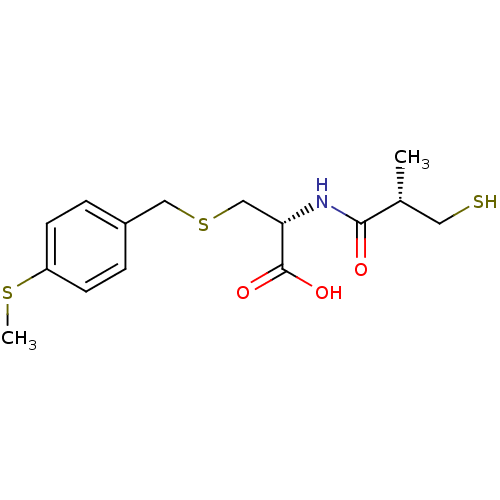
((R)-2-((S)-3-mercapto-2-methylpropanamido)-3-(4-(m...)Show SMILES CSc1ccc(CSC[C@H](NC(=O)[C@H](C)CS)C(O)=O)cc1 |r| Show InChI InChI=1S/C15H21NO3S3/c1-10(7-20)14(17)16-13(15(18)19)9-22-8-11-3-5-12(21-2)6-4-11/h3-6,10,13,20H,7-9H2,1-2H3,(H,16,17)(H,18,19)/t10-,13+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase in guinea pig lung assessed as inhibition of LTB4 production |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50272142

((2S,5S)-5-(4-isopropylbenzylthio)-1-((S)-3-mercapt...)Show SMILES CC(C)c1ccc(CS[C@H]2CC[C@H](N2C(=O)[C@H](C)CS)C(O)=O)cc1 |r| Show InChI InChI=1S/C19H27NO3S2/c1-12(2)15-6-4-14(5-7-15)11-25-17-9-8-16(19(22)23)20(17)18(21)13(3)10-24/h4-7,12-13,16-17,24H,8-11H2,1-3H3,(H,22,23)/t13-,16+,17+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig lung leukotriene A4 hydrolase |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM16314

(2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...)Show InChI InChI=1S/C16H14F3NO3S/c1-20(8-13(21)22)15(24)11-5-3-4-10-9(11)6-7-12(23-2)14(10)16(17,18)19/h3-7H,8H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged AKR1B10 expressed in Escherichia coli BL21 using retinaldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50266247
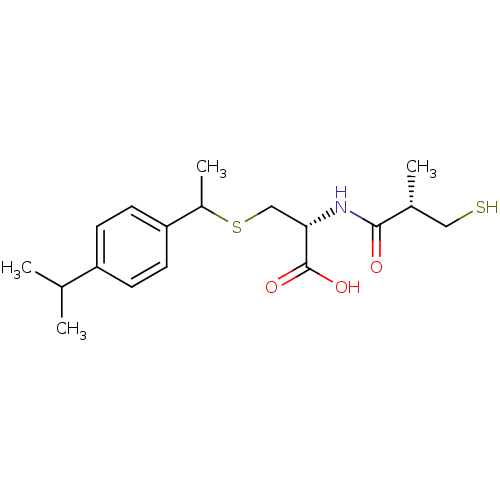
((2R)-3-(1-(4-isopropylphenyl)ethylthio)-2-((S)-3-m...)Show SMILES CC(C)c1ccc(cc1)C(C)SC[C@H](NC(=O)[C@H](C)CS)C(O)=O |r| Show InChI InChI=1S/C18H27NO3S2/c1-11(2)14-5-7-15(8-6-14)13(4)24-10-16(18(21)22)19-17(20)12(3)9-23/h5-8,11-13,16,23H,9-10H2,1-4H3,(H,19,20)(H,21,22)/t12-,13?,16+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase in guinea pig lung assessed as inhibition of LTB4 production |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50266316
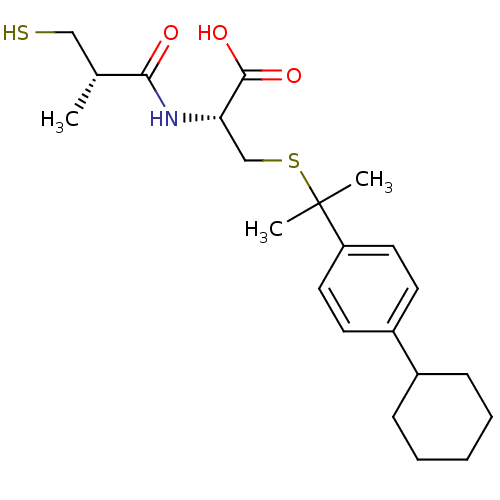
((R)-3-(2-(4-cyclohexylphenyl)propan-2-ylthio)-2-((...)Show SMILES C[C@H](CS)C(=O)N[C@@H](CSC(C)(C)c1ccc(cc1)C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C22H33NO3S2/c1-15(13-27)20(24)23-19(21(25)26)14-28-22(2,3)18-11-9-17(10-12-18)16-7-5-4-6-8-16/h9-12,15-16,19,27H,4-8,13-14H2,1-3H3,(H,23,24)(H,25,26)/t15-,19+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase in guinea pig lung assessed as inhibition of LTB4 production |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50059989
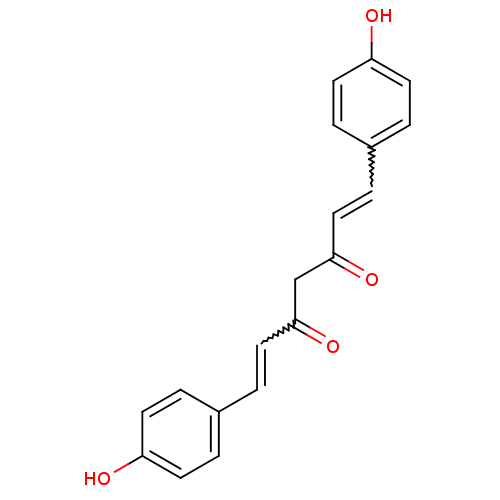
((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...)Show SMILES Oc1ccc(C=CC(=O)CC(=O)C=Cc2ccc(O)cc2)cc1 |w:12.11,5.4| Show InChI InChI=1S/C19H16O4/c20-16-7-1-14(2-8-16)5-11-18(22)13-19(23)12-6-15-3-9-17(21)10-4-15/h1-12,20-21H,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1B10 (unknown origin) |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50266248
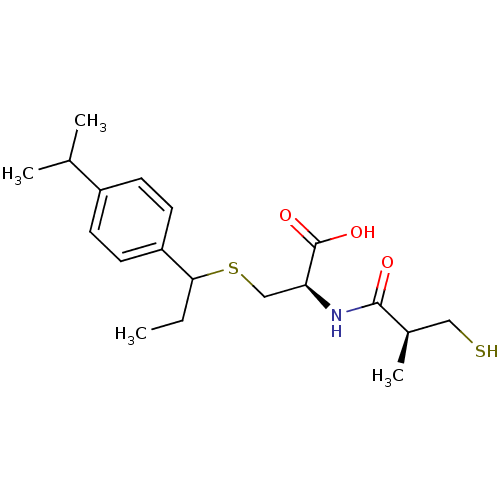
((2R)-3-(1-(4-isopropylphenyl)propylthio)-2-((S)-3-...)Show SMILES CCC(SC[C@H](NC(=O)[C@H](C)CS)C(O)=O)c1ccc(cc1)C(C)C |r| Show InChI InChI=1S/C19H29NO3S2/c1-5-17(15-8-6-14(7-9-15)12(2)3)25-11-16(19(22)23)20-18(21)13(4)10-24/h6-9,12-13,16-17,24H,5,10-11H2,1-4H3,(H,20,21)(H,22,23)/t13-,16+,17?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase in guinea pig lung assessed as inhibition of LTB4 production |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50266171
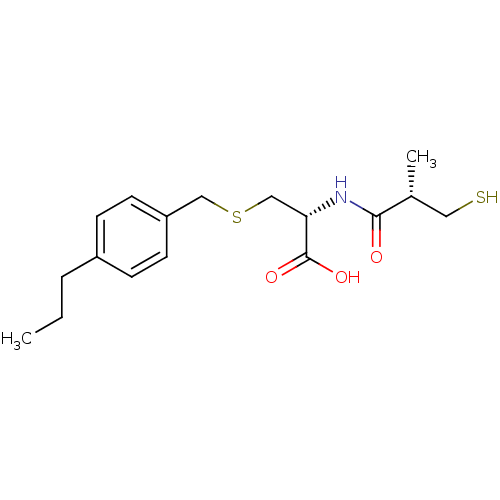
((R)-2-((S)-3-mercapto-2-methylpropanamido)-3-(4-pr...)Show SMILES CCCc1ccc(CSC[C@H](NC(=O)[C@H](C)CS)C(O)=O)cc1 |r| Show InChI InChI=1S/C17H25NO3S2/c1-3-4-13-5-7-14(8-6-13)10-23-11-15(17(20)21)18-16(19)12(2)9-22/h5-8,12,15,22H,3-4,9-11H2,1-2H3,(H,18,19)(H,20,21)/t12-,15+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase in guinea pig lung assessed as inhibition of LTB4 production |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50266175
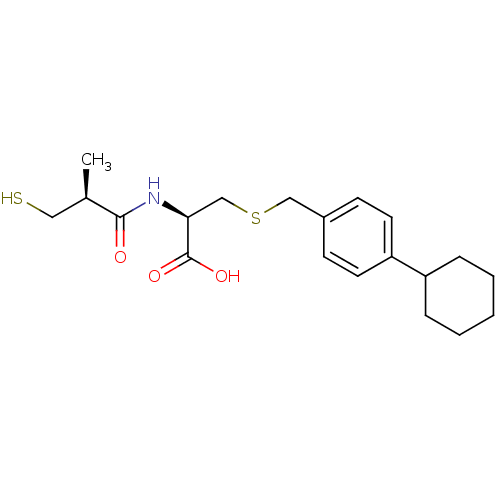
((R)-3-(4-cyclohexylbenzylthio)-2-((S)-3-mercapto-2...)Show SMILES C[C@H](CS)C(=O)N[C@@H](CSCc1ccc(cc1)C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C20H29NO3S2/c1-14(11-25)19(22)21-18(20(23)24)13-26-12-15-7-9-17(10-8-15)16-5-3-2-4-6-16/h7-10,14,16,18,25H,2-6,11-13H2,1H3,(H,21,22)(H,23,24)/t14-,18+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase in guinea pig lung assessed as inhibition of LTB4 production |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50266280
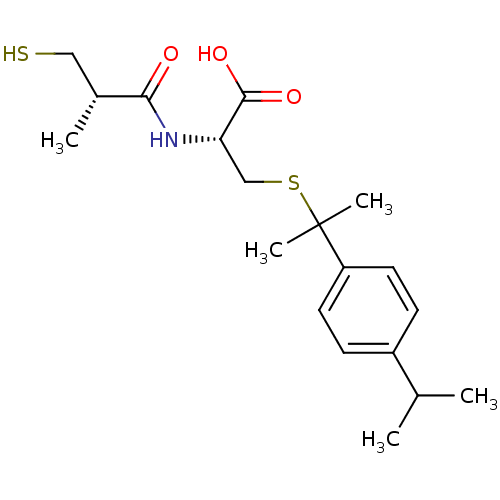
((R)-3-(2-(4-isopropylphenyl)propan-2-ylthio)-2-((S...)Show SMILES CC(C)c1ccc(cc1)C(C)(C)SC[C@H](NC(=O)[C@H](C)CS)C(O)=O |r| Show InChI InChI=1S/C19H29NO3S2/c1-12(2)14-6-8-15(9-7-14)19(4,5)25-11-16(18(22)23)20-17(21)13(3)10-24/h6-9,12-13,16,24H,10-11H2,1-5H3,(H,20,21)(H,22,23)/t13-,16+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase in guinea pig lung assessed as inhibition of LTB4 production |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM200221
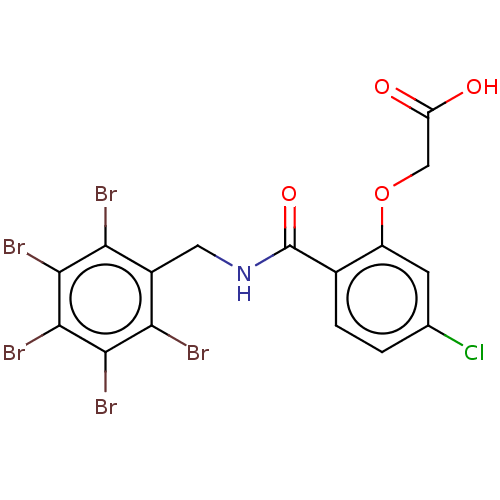
(2-(5-chloro-2-(((perbromophenyl)methyl)carbamoyl)p...)Show SMILES OC(=O)COc1cc(Cl)ccc1C(=O)NCc1c(Br)c(Br)c(Br)c(Br)c1Br Show InChI InChI=1S/C16H9Br5ClNO4/c17-11-8(12(18)14(20)15(21)13(11)19)4-23-16(26)7-2-1-6(22)3-9(7)27-5-10(24)25/h1-3H,4-5H2,(H,23,26)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1B10 expressed in Escherichia coli BL21(DE3) using pyridine-3-aldehyde as substrate measured for 3 mins by UV-vis spectrophoto... |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Cavia porcellus) | BDBM50266279
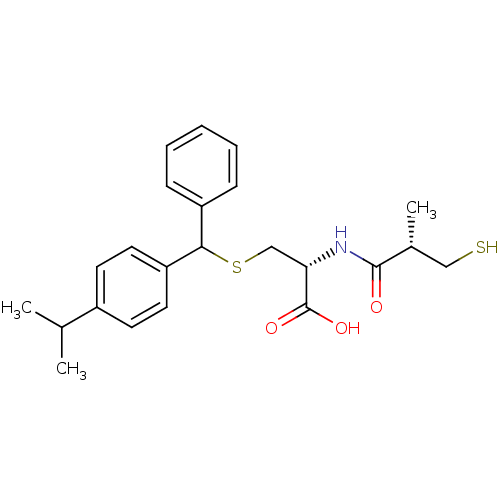
((2R)-3-((4-isopropylphenyl)(phenyl)methylthio)-2-(...)Show SMILES CC(C)c1ccc(cc1)C(SC[C@H](NC(=O)[C@H](C)CS)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C23H29NO3S2/c1-15(2)17-9-11-19(12-10-17)21(18-7-5-4-6-8-18)29-14-20(23(26)27)24-22(25)16(3)13-28/h4-12,15-16,20-21,28H,13-14H2,1-3H3,(H,24,25)(H,26,27)/t16-,20+,21?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase in guinea pig lung assessed as inhibition of LTB4 production |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241830

(CHEMBL3318218)Show InChI InChI=1S/C18H15NO5/c20-13-4-1-11(2-5-13)7-8-19-17(22)15-9-12-3-6-14(21)10-16(12)24-18(15)23/h1-6,9-10,20-21H,7-8H2,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50266133
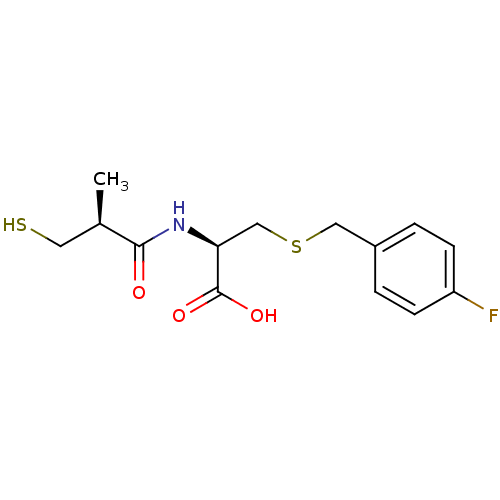
((R)-3-(4-fluorobenzylthio)-2-((S)-3-mercapto-2-met...)Show SMILES C[C@H](CS)C(=O)N[C@@H](CSCc1ccc(F)cc1)C(O)=O |r| Show InChI InChI=1S/C14H18FNO3S2/c1-9(6-20)13(17)16-12(14(18)19)8-21-7-10-2-4-11(15)5-3-10/h2-5,9,12,20H,6-8H2,1H3,(H,16,17)(H,18,19)/t9-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin-converting enzyme (unknown origin) in whole blood by HPLC |
Bioorg Med Chem Lett 19: 442-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.042
BindingDB Entry DOI: 10.7270/Q20K28FC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data