| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Leukotriene A-4 hydrolase |
|---|
| Ligand | BDBM50266316 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_544515 (CHEMBL1013487) |
|---|
| IC50 | 55±n/a nM |
|---|
| Citation |  Enomoto, H; Morikawa, Y; Miyake, Y; Tsuji, F; Mizuchi, M; Suhara, H; Fujimura, K; Horiuchi, M; Ban, M Synthesis and biological evaluation of N-mercaptoacylcysteine derivatives as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett19:442-6 (2008) [PubMed] Article Enomoto, H; Morikawa, Y; Miyake, Y; Tsuji, F; Mizuchi, M; Suhara, H; Fujimura, K; Horiuchi, M; Ban, M Synthesis and biological evaluation of N-mercaptoacylcysteine derivatives as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett19:442-6 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Leukotriene A-4 hydrolase |
|---|
| Name: | Leukotriene A-4 hydrolase |
|---|
| Synonyms: | LKHA4_CAVPO | LTA4H |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 68972.78 |
|---|
| Organism: | Cavia porcellus |
|---|
| Description: | ChEMBL_544515 |
|---|
| Residue: | 611 |
|---|
| Sequence: | MPEVVDTCSLASPATVCRTKHLHLRCSVDFTRRALTGVAALTIQSQEDNLRSLILDTKDL
TIEKVVINGQEVKYALGEKQSYKGSPMEISLPIALSKNQEVVIEISFETSPKSSALQWLT
PEQTSGKEHPYLFSQCQAIHCRAFLPCQDTPSVKLTYTAEVSVPKELVALMSAIRDGEAP
DPADPSRKIYKFSQKVPIPCYLIALVVGALESRKIGPRTLVWSEKEQVDKSAYEFSETES
MLKIAEDLGGPYVWGQYDRLVLPPSFSYGGMENPCLTFVTPTLLAGDKSLSNVIAHEISH
TWTGNLVTNKTWDHFWLNEGHTVYLERHICGRLFGEKFRHFHALGGWGELQNTVKTLGET
QAFTKLVVDLTDTDPDVAYSSVPYEKGFALLFHLEQLLGGPEVFLGFLKAYVEKFSYKSI
TTDDWKNFLFSHFKDKVDILNQVDWDAWLYSPGLPPIKPNYDMTLTNACIALSQRWITAK
EKDLNTFSATDLKDLSSHQVNEFLAQVLQRAPLPLGHVKRMQEVYNCNAINNSEIRFRWL
RLCIQSKWEEAIPLALKMATEQGRMKFTRPLFKDLAAFDKSHDQAIQTYHAHKASMHPVT
AMLVGKDLKVE
|
|
|
|---|
| BDBM50266316 |
|---|
| n/a |
|---|
| Name | BDBM50266316 |
|---|
| Synonyms: | (R)-3-(2-(4-cyclohexylphenyl)propan-2-ylthio)-2-((S)-3-mercapto-2-methylpropanamido)propanoic acid | CHEMBL456045 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H33NO3S2 |
|---|
| Mol. Mass. | 423.632 |
|---|
| SMILES | C[C@H](CS)C(=O)N[C@@H](CSC(C)(C)c1ccc(cc1)C1CCCCC1)C(O)=O |r| |
|---|
| Structure | 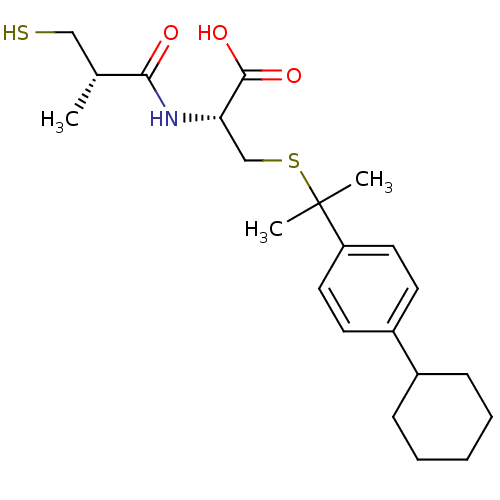
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Enomoto, H; Morikawa, Y; Miyake, Y; Tsuji, F; Mizuchi, M; Suhara, H; Fujimura, K; Horiuchi, M; Ban, M Synthesis and biological evaluation of N-mercaptoacylcysteine derivatives as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett19:442-6 (2008) [PubMed] Article
Enomoto, H; Morikawa, Y; Miyake, Y; Tsuji, F; Mizuchi, M; Suhara, H; Fujimura, K; Horiuchi, M; Ban, M Synthesis and biological evaluation of N-mercaptoacylcysteine derivatives as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett19:442-6 (2008) [PubMed] Article