| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Platelet-activating factor receptor |
|---|
| Ligand | BDBM50286012 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_155162 |
|---|
| Ki | 2.3±n/a nM |
|---|
| Citation |  Davidsen, SK; Summers, JB; Sweeny, DJ; Holms, JH; Albert, DH; Carrera, GM; Tapang, P; Magoc, TJ; Conway, RG; Rhein, DA Synthesis of active metabolites of indole pyrrolothiazole paf antagonists Bioorg Med Chem Lett5:2909-2912 (1995) Article Davidsen, SK; Summers, JB; Sweeny, DJ; Holms, JH; Albert, DH; Carrera, GM; Tapang, P; Magoc, TJ; Conway, RG; Rhein, DA Synthesis of active metabolites of indole pyrrolothiazole paf antagonists Bioorg Med Chem Lett5:2909-2912 (1995) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Platelet-activating factor receptor |
|---|
| Name: | Platelet-activating factor receptor |
|---|
| Synonyms: | PAF Platelet activating factor | PAF-R | PTAFR_RAT | Platelet activating factor receptor | Platelet-activating factor receptor | Ptafr |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 39221.53 |
|---|
| Organism: | RAT |
|---|
| Description: | PAF Platelet activating factor PTAFR RAT::P46002 |
|---|
| Residue: | 341 |
|---|
| Sequence: | MEQNGSFRVDSEFRYTLFPIVYSVIFVLGVVANGYVLWVFATLYPSKKLNEIKIFMVNLT
VADLLFLMTLPLWIVYYSNEGDWIVHKFLCNLAGCLFFINTYCSVAFLGVITYNRYQAVA
YPIKTAQATTRKRGITLSLVIWISIAATASYFLATDSTNVVPKKDGSGNITRCFEHYEPY
SVPILVVHIFITSCFFLVFFLIFYCNMVIIHTLLTRPVRQQRKPEVKRRALWMVCTVLAV
FVICFVPHHVVQLPWTLAELGYQTNFHQAINDAHQITLCLLSTNCVLDPVIYCFLTKKFR
KHLSEKFYSMRSSRKCSRATSDTCTEVMMPANQTPVLPLKN
|
|
|
|---|
| BDBM50286012 |
|---|
| n/a |
|---|
| Name | BDBM50286012 |
|---|
| Synonyms: | 3-{(R)-7-[1-Dimethylcarbamoyl-6-(4-fluoro-phenyl)-1H-indole-3-carbonyl]-1H-pyrrolo[1,2-c]thiazol-3-yl}-1-hydroxy-pyridinium | 6-(4-Fluoro-phenyl)-3-[(R)-3-(1-oxy-pyridin-3-yl)-1H-pyrrolo[1,2-c]thiazole-7-carbonyl]-indole-1-carboxylic acid dimethylamide | CHEMBL317745 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H23FN4O3S |
|---|
| Mol. Mass. | 526.581 |
|---|
| SMILES | CN(C)C(=O)n1cc(C(=O)c2ccn3[C@H](SCc23)c2ccc[n+]([O-])c2)c2ccc(cc12)-c1ccc(F)cc1 |
|---|
| Structure | 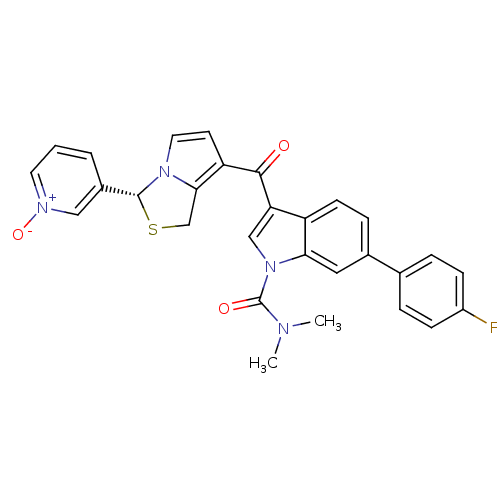
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Davidsen, SK; Summers, JB; Sweeny, DJ; Holms, JH; Albert, DH; Carrera, GM; Tapang, P; Magoc, TJ; Conway, RG; Rhein, DA Synthesis of active metabolites of indole pyrrolothiazole paf antagonists Bioorg Med Chem Lett5:2909-2912 (1995) Article
Davidsen, SK; Summers, JB; Sweeny, DJ; Holms, JH; Albert, DH; Carrera, GM; Tapang, P; Magoc, TJ; Conway, RG; Rhein, DA Synthesis of active metabolites of indole pyrrolothiazole paf antagonists Bioorg Med Chem Lett5:2909-2912 (1995) Article