| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50305878 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_605302 (CHEMBL1071327) |
|---|
| IC50 | 50000±n/a nM |
|---|
| Citation |  Lin, H; Yamashita, DS; Zeng, J; Xie, R; Verma, S; Luengo, JI; Rhodes, N; Zhang, S; Robell, KA; Choudhry, AE; Lai, Z; Kumar, R; Minthorn, EA; Brown, KK; Heerding, DA 2,3,5-Trisubstituted pyridines as selective AKT inhibitors. Part II: Improved drug-like properties and kinase selectivity from azaindazoles. Bioorg Med Chem Lett20:679-83 (2010) [PubMed] Article Lin, H; Yamashita, DS; Zeng, J; Xie, R; Verma, S; Luengo, JI; Rhodes, N; Zhang, S; Robell, KA; Choudhry, AE; Lai, Z; Kumar, R; Minthorn, EA; Brown, KK; Heerding, DA 2,3,5-Trisubstituted pyridines as selective AKT inhibitors. Part II: Improved drug-like properties and kinase selectivity from azaindazoles. Bioorg Med Chem Lett20:679-83 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50305878 |
|---|
| n/a |
|---|
| Name | BDBM50305878 |
|---|
| Synonyms: | (2S)-1-{[6-furan-3-yl-5-(3-methyl-2H-indazol-5-yl)pyridin-3-yl]oxy}-3-(1H-indol-3-yl)propan-2-amine | (S)-1-(6-(furan-3-yl)-5-(3-methyl-1H-indazol-5-yl)pyridin-3-yloxy)-3-(1H-indol-3-yl)propan-2-amine | CHEMBL593490 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H25N5O2 |
|---|
| Mol. Mass. | 463.5304 |
|---|
| SMILES | Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)cnc1-c1ccoc1 |r| |
|---|
| Structure | 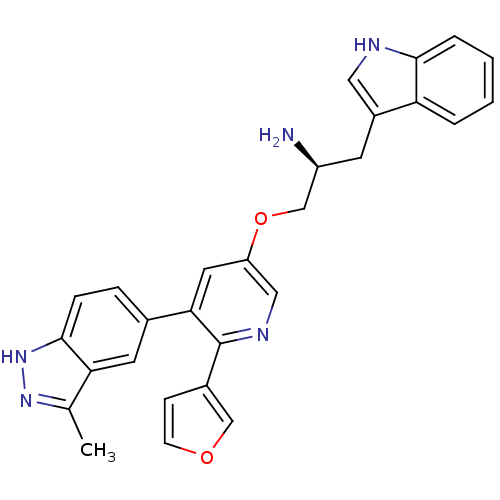
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lin, H; Yamashita, DS; Zeng, J; Xie, R; Verma, S; Luengo, JI; Rhodes, N; Zhang, S; Robell, KA; Choudhry, AE; Lai, Z; Kumar, R; Minthorn, EA; Brown, KK; Heerding, DA 2,3,5-Trisubstituted pyridines as selective AKT inhibitors. Part II: Improved drug-like properties and kinase selectivity from azaindazoles. Bioorg Med Chem Lett20:679-83 (2010) [PubMed] Article
Lin, H; Yamashita, DS; Zeng, J; Xie, R; Verma, S; Luengo, JI; Rhodes, N; Zhang, S; Robell, KA; Choudhry, AE; Lai, Z; Kumar, R; Minthorn, EA; Brown, KK; Heerding, DA 2,3,5-Trisubstituted pyridines as selective AKT inhibitors. Part II: Improved drug-like properties and kinase selectivity from azaindazoles. Bioorg Med Chem Lett20:679-83 (2010) [PubMed] Article