| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-X-C chemokine receptor type 2 |
|---|
| Ligand | BDBM50315303 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_626222 (CHEMBL1107989) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Skerlj, RT; Bridger, GJ; Kaller, A; McEachern, EJ; Crawford, JB; Zhou, Y; Atsma, B; Langille, J; Nan, S; Veale, D; Wilson, T; Harwig, C; Hatse, S; Princen, K; De Clercq, E; Schols, D Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J Med Chem53:3376-88 (2010) [PubMed] Article Skerlj, RT; Bridger, GJ; Kaller, A; McEachern, EJ; Crawford, JB; Zhou, Y; Atsma, B; Langille, J; Nan, S; Veale, D; Wilson, T; Harwig, C; Hatse, S; Princen, K; De Clercq, E; Schols, D Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J Med Chem53:3376-88 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-X-C chemokine receptor type 2 |
|---|
| Name: | C-X-C chemokine receptor type 2 |
|---|
| Synonyms: | C-X-C chemokine receptor type 2 (CXCR-2) | C-X-C chemokine receptor type 2 (CXCR2) | CD_antigen=CD182 | CDw128b | CXCR-2 | CXCR2 | CXCR2_HUMAN | Chemokine receptor type 2 (CXCR2) | GRO/MGSA receptor | High affinity interleukin-8 receptor B | IL-8 receptor type 2 | IL-8R B | IL8RB | Interleukin-8 receptor B |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 40767.88 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P25025 |
|---|
| Residue: | 360 |
|---|
| Sequence: | MEDFNMESDSFEDFWKGEDLSNYSYSSTLPPFLLDAAPCEPESLEINKYFVVIIYALVFL
LSLLGNSLVMLVILYSRVGRSVTDVYLLNLALADLLFALTLPIWAASKVNGWIFGTFLCK
VVSLLKEVNFYSGILLLACISVDRYLAIVHATRTLTQKRYLVKFICLSIWGLSLLLALPV
LLFRRTVYSSNVSPACYEDMGNNTANWRMLLRILPQSFGFIVPLLIMLFCYGFTLRTLFK
AHMGQKHRAMRVIFAVVLIFLLCWLPYNLVLLADTLMRTQVIQETCERRNHIDRALDATE
ILGILHSCLNPLIYAFIGQKFRHGLLKILAIHGLISKDSLPKDSRPSFVGSSSGHTSTTL
|
|
|
|---|
| BDBM50315303 |
|---|
| n/a |
|---|
| Name | BDBM50315303 |
|---|
| Synonyms: | (S)-N-(2-Pyridinylmethyl)-N'-(1H-benzimidazol-2-ylmethyl)-N'-(5,6,7,8-tetrahydro-8-quinolinyl)-1,4-benzenedimethanamine | CHEMBL1093137 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H32N6 |
|---|
| Mol. Mass. | 488.626 |
|---|
| SMILES | C(NCc1ccccn1)c1ccc(CN(Cc2nc3ccccc3[nH]2)[C@H]2CCCc3cccnc23)cc1 |r| |
|---|
| Structure | 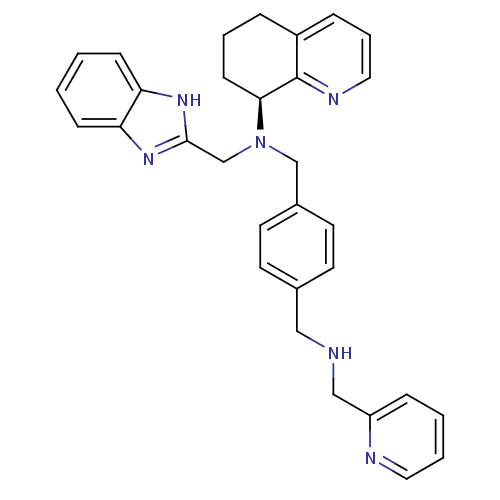
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Skerlj, RT; Bridger, GJ; Kaller, A; McEachern, EJ; Crawford, JB; Zhou, Y; Atsma, B; Langille, J; Nan, S; Veale, D; Wilson, T; Harwig, C; Hatse, S; Princen, K; De Clercq, E; Schols, D Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J Med Chem53:3376-88 (2010) [PubMed] Article
Skerlj, RT; Bridger, GJ; Kaller, A; McEachern, EJ; Crawford, JB; Zhou, Y; Atsma, B; Langille, J; Nan, S; Veale, D; Wilson, T; Harwig, C; Hatse, S; Princen, K; De Clercq, E; Schols, D Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J Med Chem53:3376-88 (2010) [PubMed] Article