

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-2-related protein A1 (Homo sapiens (Human)) | BDBM50210070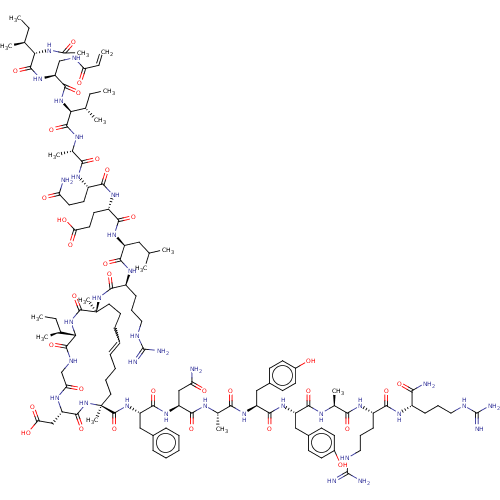 (CHEMBL3883565) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of FITC-betaA-DIIRNIARHLAQVGDSMRSI-NH2 binding to recombinant human Bcl2A1 (1 to 152 residues) BH3 binding site expressed in Escherichia c... | ACS Med Chem Lett 8: 22-26 (2017) Article DOI: 10.1021/acsmedchemlett.6b00395 BindingDB Entry DOI: 10.7270/Q2NS0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270276 (CHEMBL477121 | N-[1,4,8,11-Tetraazacyclotetradecan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human wild type CXCR4 expressed in COS7 cells coexpressing G protein Gqi4myr assessed as inhibition of CXCL12-induced phosphat... | J Biol Chem 282: 27354-65 (2007) Article DOI: 10.1074/jbc.M704739200 BindingDB Entry DOI: 10.7270/Q26Q1X1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-related protein A1 (Homo sapiens (Human)) | BDBM50210069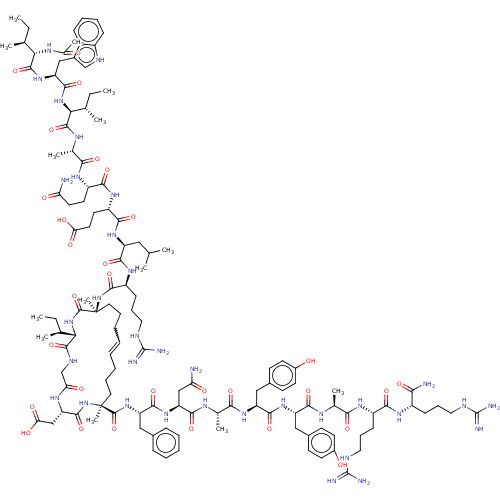 (CHEMBL3884841) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of FITC-betaA-DIIRNIARHLAQVGDSMRSI-NH2 binding to recombinant human Bcl2A1 (1 to 152 residues) BH3 binding site expressed in Escherichia c... | ACS Med Chem Lett 8: 22-26 (2017) Article DOI: 10.1021/acsmedchemlett.6b00395 BindingDB Entry DOI: 10.7270/Q2NS0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270276 (CHEMBL477121 | N-[1,4,8,11-Tetraazacyclotetradecan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [125I]12G5 antibody from human wild type CXCR4 expressed in COS7 cells | J Biol Chem 282: 27354-65 (2007) Article DOI: 10.1074/jbc.M704739200 BindingDB Entry DOI: 10.7270/Q26Q1X1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035696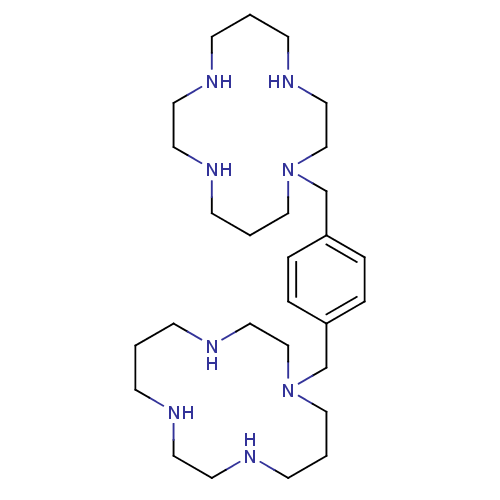 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human wild type CXCR4 expressed in COS7 cells coexpressing G protein Gqi4myr assessed as inhibition of CXCL12-induced phosphat... | J Biol Chem 282: 27354-65 (2007) Article DOI: 10.1074/jbc.M704739200 BindingDB Entry DOI: 10.7270/Q26Q1X1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035696 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [125I]12G5 antibody from human wild type CXCR4 expressed in COS7 cells | J Biol Chem 282: 27354-65 (2007) Article DOI: 10.1074/jbc.M704739200 BindingDB Entry DOI: 10.7270/Q26Q1X1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270277 (Benzyl-[4-(1,4,8,11tetraaza-cyclotetradec-1-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human wild type CXCR4 expressed in COS7 cells coexpressing G protein Gqi4myr assessed as inhibition of CXCL12-induced phosphat... | J Biol Chem 282: 27354-65 (2007) Article DOI: 10.1074/jbc.M704739200 BindingDB Entry DOI: 10.7270/Q26Q1X1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035719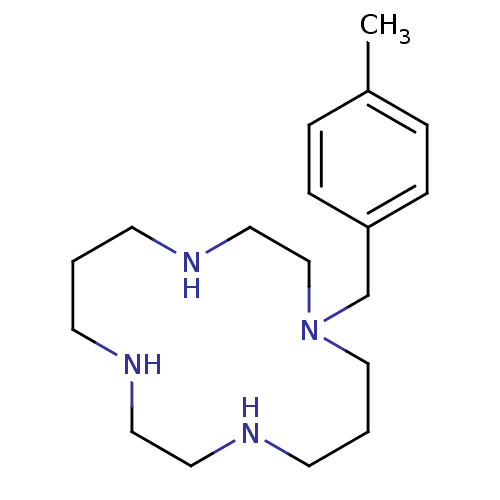 (1-(4-Methyl-benzyl)-1,4,8,11tetraaza-cyclotetradec...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at human wild type CXCR4 expressed in COS7 cells coexpressing G protein Gqi4myr assessed as inhibition of CXCL12-induced phosphat... | J Biol Chem 282: 27354-65 (2007) Article DOI: 10.1074/jbc.M704739200 BindingDB Entry DOI: 10.7270/Q26Q1X1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50369468 (CHEMBL1202231) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AnorMED Inc. Curated by ChEMBL | Assay Description Inhibitory activity against CX3C chemokine receptor 4-specific monoclonal antibody 12G5 (mAb-12G5) binding to human chemokinin receptor CXCR4 in lymp... | J Med Chem 42: 3971-81 (1999) BindingDB Entry DOI: 10.7270/Q2K0750M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035696 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AnorMED Inc. Curated by ChEMBL | Assay Description Inhibitory activity against CX3C chemokine receptor 4-specific monoclonal antibody 12G5 (mAb-12G5) binding to human chemokinin receptor CXCR4 in lymp... | J Med Chem 42: 3971-81 (1999) BindingDB Entry DOI: 10.7270/Q2K0750M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-related protein A1 (Homo sapiens (Human)) | BDBM50210070 (CHEMBL3883565) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of FITC-betaA-DIIRNIARHLAQVGDSMRSI-NH2 binding to recombinant human Bcl2A1 (1 to 152 residues) BH3 binding site expressed in Escherichia c... | ACS Med Chem Lett 8: 22-26 (2017) Article DOI: 10.1021/acsmedchemlett.6b00395 BindingDB Entry DOI: 10.7270/Q2NS0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315287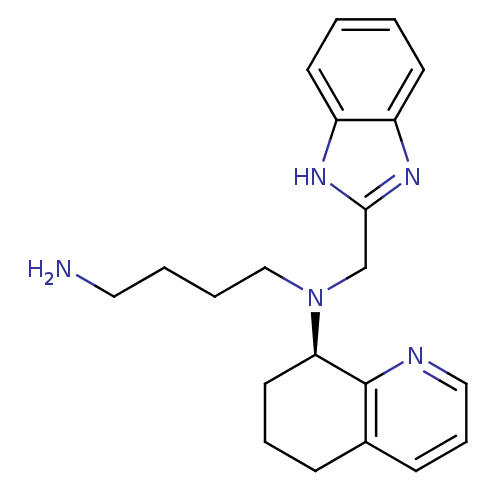 ((R)-N1-((1H-benzo[d]imidazol-2-yl)methyl)-N1-(5,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315305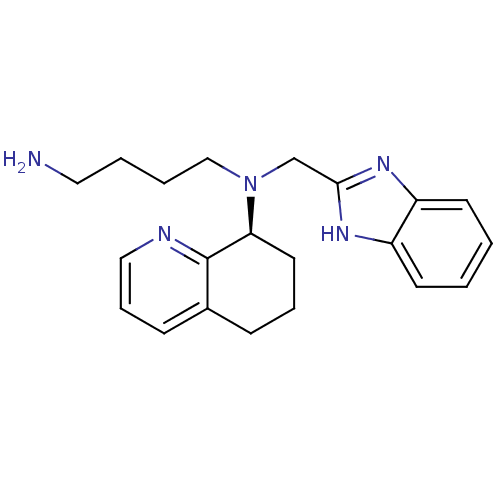 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human CEM-CCRF cells by liquid scintillation counting | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315287 ((R)-N1-((1H-benzo[d]imidazol-2-yl)methyl)-N1-(5,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human CEM-CCRF cells by liquid scintillation counting | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315290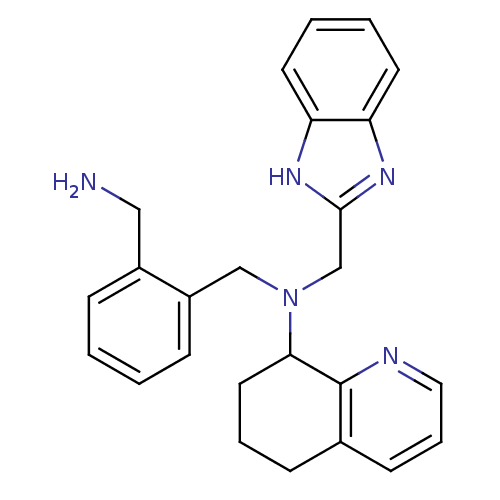 (CHEMBL1093008 | N-[(1H-Benzo[d]midazol-2-yl)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50081406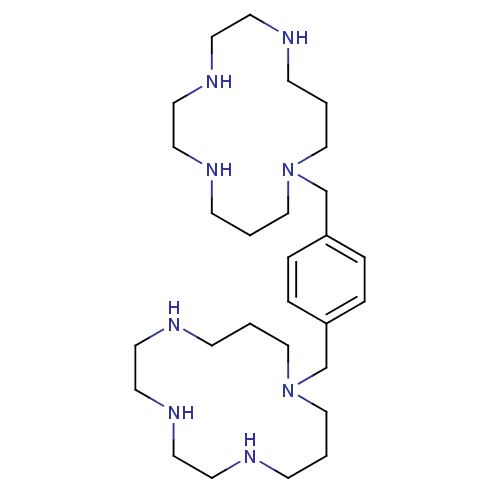 (1,5,8,11-tetraazacyclotetradecanyl[4-(1,5,8,11-tet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
AnorMED Inc. Curated by ChEMBL | Assay Description Inhibitory activity against CX3C chemokine receptor 4-specific monoclonal antibody 12G5 (mAb-12G5) binding to human chemokinin receptor CXCR4 in lymp... | J Med Chem 42: 3971-81 (1999) BindingDB Entry DOI: 10.7270/Q2K0750M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315295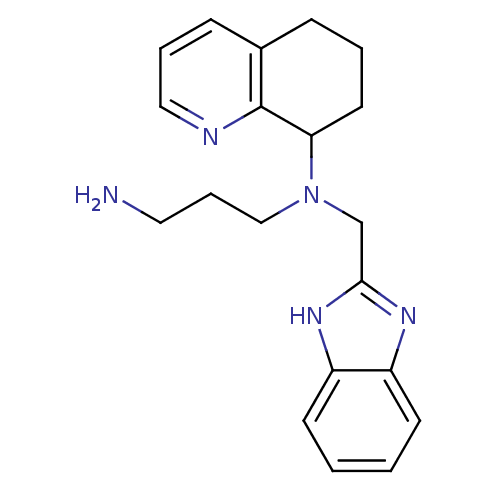 (CHEMBL1088912 | N1-(1H-Benzo[d]imidazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315297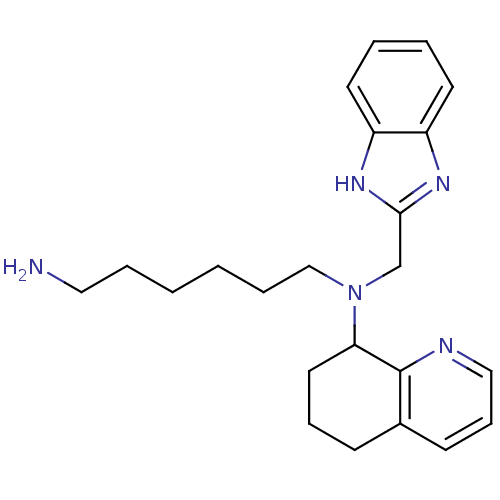 (CHEMBL1088916 | N1-(1H-Benzo[d]imidazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315291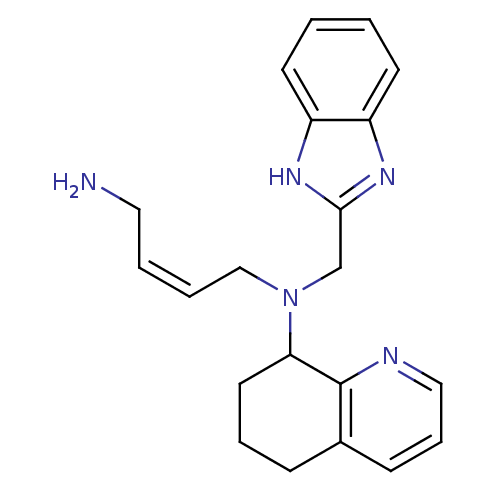 ((Z)-N1-((1H-Benzo[d]imidazol-2-yl)methyl)-N1-(5,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315298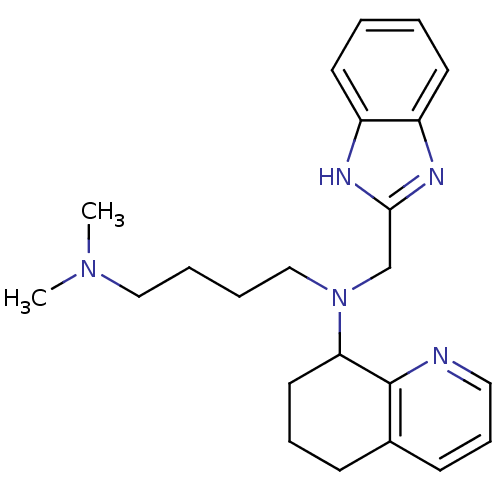 (CHEMBL1092301 | N1-(1H-Benzo[d]imidazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50379157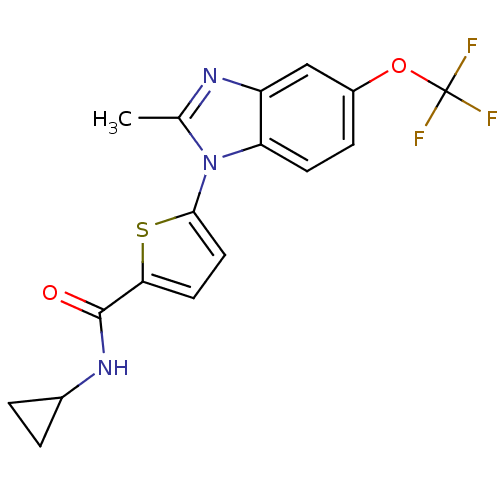 (CHEMBL1234899 | US8703811, 57) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 using bufuralol as substrate after 10 mins by LC-MS/MS analysis | ACS Med Chem Lett 2: 708-713 (2011) Article DOI: 10.1021/ml200143c BindingDB Entry DOI: 10.7270/Q2C24XDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-related protein A1 (Homo sapiens (Human)) | BDBM50210069 (CHEMBL3884841) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of FITC-betaA-DIIRNIARHLAQVGDSMRSI-NH2 binding to recombinant human Bcl2A1 (1 to 152 residues) BH3 binding site expressed in Escherichia c... | ACS Med Chem Lett 8: 22-26 (2017) Article DOI: 10.1021/acsmedchemlett.6b00395 BindingDB Entry DOI: 10.7270/Q2NS0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315288 (CHEMBL1092324 | N-[(1H-Benzo[d]imidazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315303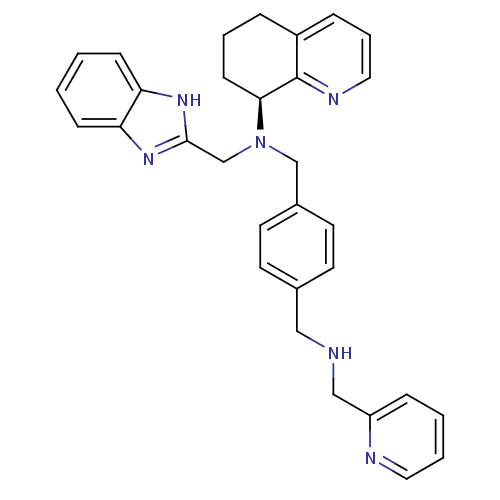 ((S)-N-(2-Pyridinylmethyl)-N'-(1H-benzimidazol-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of anti-CXCR4 12G5 monoclonal antibody to CXCR4 in human SUP-T1 cells pretreated for 30 mins by flow cytometry | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315292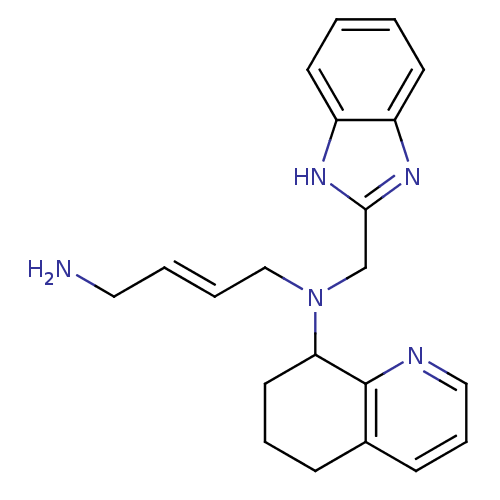 ((E)-N1-((1H-Benzo[d]imidazol-2-yl)methyl)-N1-(5,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315299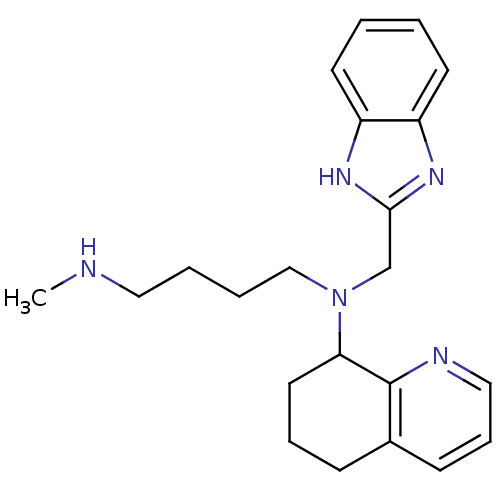 (CHEMBL1092302 | N1-(1H-Benzo[d]imidazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315287 ((R)-N1-((1H-benzo[d]imidazol-2-yl)methyl)-N1-(5,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human CEM-CCRF cells by liquid scintillation counting | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315302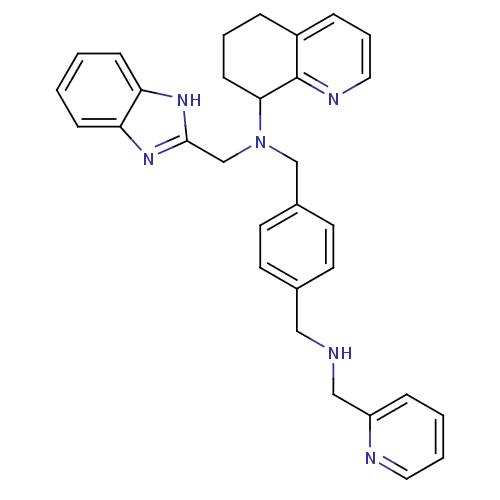 (CHEMBL1089434 | N-(2-Pyridinylmethyl)-N'-(1H-benzi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Inhibition of anti-CXCR4 12G5 monoclonal antibody to CXCR4 in human SUP-T1 cells pretreated for 30 mins by flow cytometry | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315300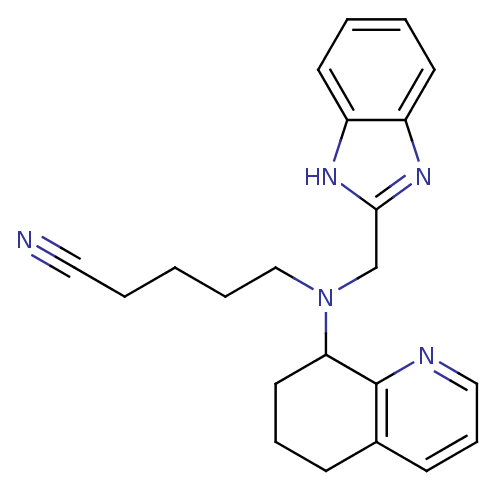 (5-(((1H-Benzo[d]imidazol-2-yl)methyl)(5,6,7,8-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 442 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315296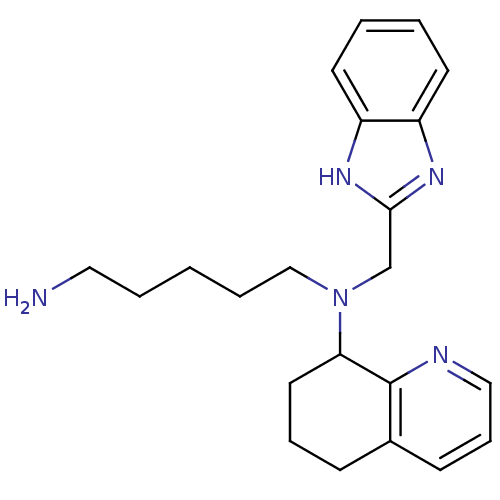 (CHEMBL1092596 | N1-(1H-Benzo[d]imidazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 514 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315293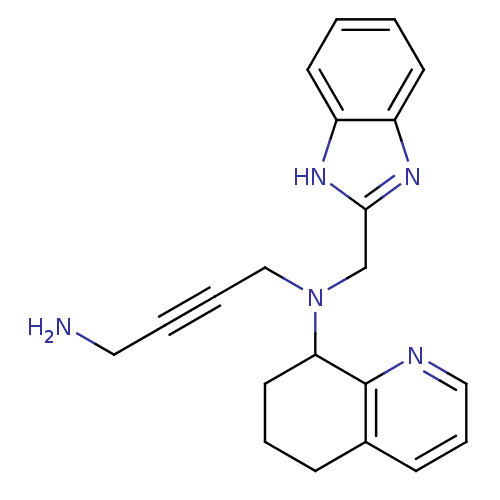 (CHEMBL1089846 | N1-(1H-Benzo[d]imidazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 973 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315294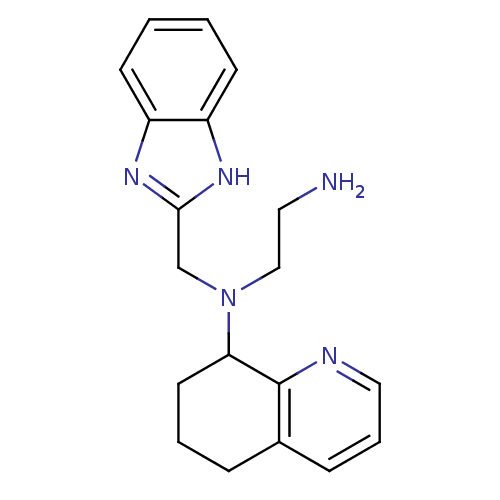 (CHEMBL1093302 | N1-(1H-Benzo[d]imidazol-2-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315289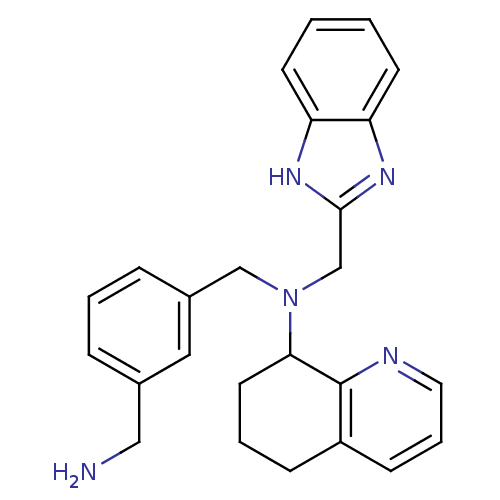 (CHEMBL1088867 | N-[(1H-Benzo[d]midazol-2-yl)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50379150 (CHEMBL2012961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assay | ACS Med Chem Lett 2: 708-713 (2011) Article DOI: 10.1021/ml200143c BindingDB Entry DOI: 10.7270/Q2C24XDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315301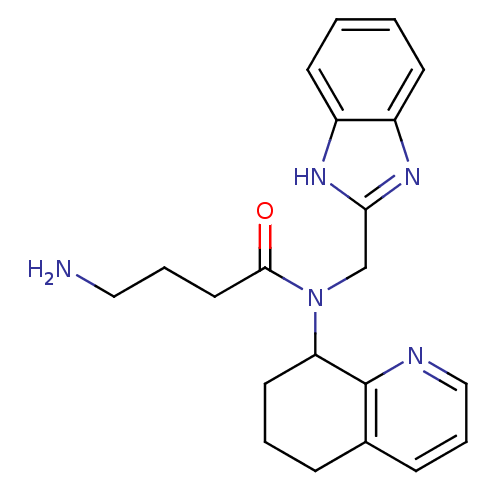 (CHEMBL1092629 | N-((1H-Benzo[d]imidazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50369469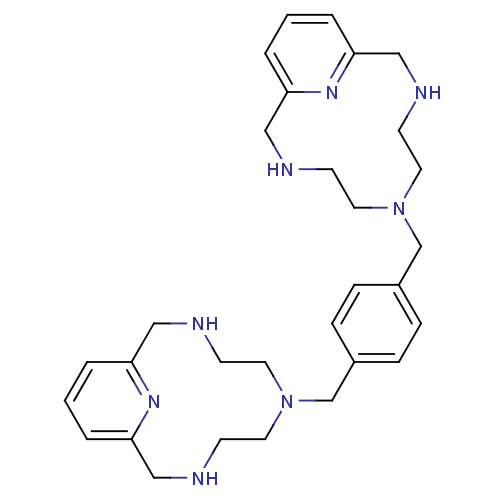 (CHEMBL1202230) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AnorMED Inc. Curated by ChEMBL | Assay Description Inhibitory activity against CX3C chemokine receptor 4-specific monoclonal antibody 12G5 (mAb-12G5) binding to human chemokinin receptor CXCR4 in lymp... | J Med Chem 42: 3971-81 (1999) BindingDB Entry DOI: 10.7270/Q2K0750M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50379152 (CHEMBL2012963) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assay | ACS Med Chem Lett 2: 708-713 (2011) Article DOI: 10.1021/ml200143c BindingDB Entry DOI: 10.7270/Q2C24XDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50081405 (15-chloro-3,7,11,17-tetraazabicyclo[11.3.1]heptade...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AnorMED Inc. Curated by ChEMBL | Assay Description Inhibitory activity against CX3C chemokine receptor 4-specific monoclonal antibody 12G5 (mAb-12G5) binding to human chemokinin receptor CXCR4 in lymp... | J Med Chem 42: 3971-81 (1999) BindingDB Entry DOI: 10.7270/Q2K0750M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50379153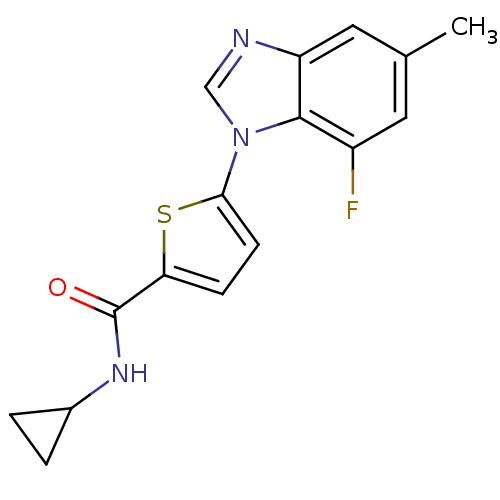 (CHEMBL2012964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human DHODH after 20 mins by 2,6-dichloroindophenol-reduction based assay | ACS Med Chem Lett 2: 708-713 (2011) Article DOI: 10.1021/ml200143c BindingDB Entry DOI: 10.7270/Q2C24XDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50315305 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of radiolabeled IL8 from CXCR2 receptor | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50315303 ((S)-N-(2-Pyridinylmethyl)-N'-(1H-benzimidazol-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of radiolabeled IL8 from CXCR2 receptor | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50315305 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of radiolabeled IL8 from CXCR1 receptor | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50315303 ((S)-N-(2-Pyridinylmethyl)-N'-(1H-benzimidazol-2-yl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of radiolabeled IL8 from CXCR1 receptor | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50315305 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of radiolabeled MIP1beta from CCR5 receptor | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50315303 ((S)-N-(2-Pyridinylmethyl)-N'-(1H-benzimidazol-2-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of radiolabeled MIP1beta from CCR5 receptor | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315305 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of radiolabeled TARC from CCR4 receptor | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315303 ((S)-N-(2-Pyridinylmethyl)-N'-(1H-benzimidazol-2-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of radiolabeled TARC from CCR4 receptor | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50379138 (CHEMBL2012951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 using phenacetin as substrate after 10 mins by LC-MS/MS analysis | ACS Med Chem Lett 2: 708-713 (2011) Article DOI: 10.1021/ml200143c BindingDB Entry DOI: 10.7270/Q2C24XDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50315303 ((S)-N-(2-Pyridinylmethyl)-N'-(1H-benzimidazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of radiolabeled MCP1 from CCR2b receptor | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50315305 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of radiolabeled MIP1alpha from CCR1 receptor | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 85 total ) | Next | Last >> |