| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2a |
|---|
| Ligand | BDBM50316885 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_629845 (CHEMBL1116482) |
|---|
| Ki | 13.7±n/a nM |
|---|
| Citation |  Shook, BC; Rassnick, S; Chakravarty, D; Wallace, N; Ault, M; Crooke, J; Barbay, JK; Wang, A; Leonard, K; Powell, MT; Alford, V; Hall, D; Rupert, KC; Heintzelman, GR; Hansen, K; Bullington, JL; Scannevin, RH; Carroll, K; Lampron, L; Westover, L; Russell, R; Branum, S; Wells, K; Damon, S; Youells, S; Beauchamp, D; Li, X; Rhodes, K; Jackson, PF Optimization of arylindenopyrimidines as potent adenosine A(2A)/A(1) antagonists. Bioorg Med Chem Lett20:2868-71 (2010) [PubMed] Article Shook, BC; Rassnick, S; Chakravarty, D; Wallace, N; Ault, M; Crooke, J; Barbay, JK; Wang, A; Leonard, K; Powell, MT; Alford, V; Hall, D; Rupert, KC; Heintzelman, GR; Hansen, K; Bullington, JL; Scannevin, RH; Carroll, K; Lampron, L; Westover, L; Russell, R; Branum, S; Wells, K; Damon, S; Youells, S; Beauchamp, D; Li, X; Rhodes, K; Jackson, PF Optimization of arylindenopyrimidines as potent adenosine A(2A)/A(1) antagonists. Bioorg Med Chem Lett20:2868-71 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2a |
|---|
| Name: | Adenosine receptor A2a |
|---|
| Synonyms: | A2A adenosine receptor (hA2A) | AA2AR_HUMAN | ADENOSINE A2 | ADENOSINE A2a | ADORA2 | ADORA2A | Adenosine A2A receptor (A2AAR) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 44716.46 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P29274 |
|---|
| Residue: | 412 |
|---|
| Sequence: | MPIMGSSVYITVELAIAVLAILGNVLVCWAVWLNSNLQNVTNYFVVSLAAADIAVGVLAI
PFAITISTGFCAACHGCLFIACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGTR
AKGIIAICWVLSFAIGLTPMLGWNNCGQPKEGKNHSQGCGEGQVACLFEDVVPMNYMVYF
NFFACVLVPLLLMLGVYLRIFLAARRQLKQMESQPLPGERARSTLQKEVHAAKSLAIIVG
LFALCWLPLHIINCFTFFCPDCSHAPLWLMYLAIVLSHTNSVVNPFIYAYRIREFRQTFR
KIIRSHVLRQQEPFKAAGTSARVLAAHGSDGEQVSLRLNGHPPGVWANGSAPHPERRPNG
YALGLVSGGSAQESQGNTGLPDVELLSHELKGVCPEPPGLDDPLAQDGAGVS
|
|
|
|---|
| BDBM50316885 |
|---|
| n/a |
|---|
| Name | BDBM50316885 |
|---|
| Synonyms: | 2-amino-8-(2-(diisopropylamino)ethoxy)-4-phenyl-5H-indeno[1,2-d]pyrimidin-5-one | CHEMBL1094721 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H28N4O2 |
|---|
| Mol. Mass. | 416.5154 |
|---|
| SMILES | CC(C)N(CCOc1ccc2C(=O)c3c(nc(N)nc3-c3ccccc3)-c2c1)C(C)C |
|---|
| Structure | 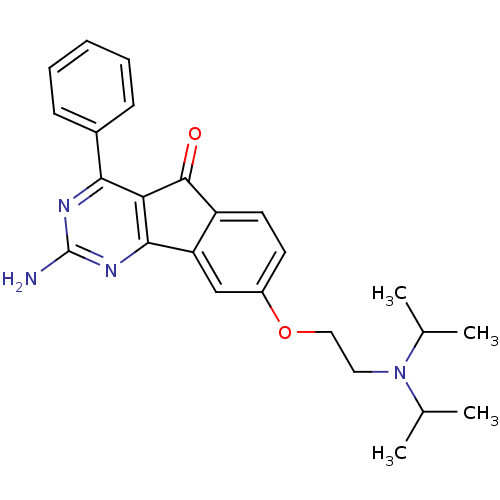
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Shook, BC; Rassnick, S; Chakravarty, D; Wallace, N; Ault, M; Crooke, J; Barbay, JK; Wang, A; Leonard, K; Powell, MT; Alford, V; Hall, D; Rupert, KC; Heintzelman, GR; Hansen, K; Bullington, JL; Scannevin, RH; Carroll, K; Lampron, L; Westover, L; Russell, R; Branum, S; Wells, K; Damon, S; Youells, S; Beauchamp, D; Li, X; Rhodes, K; Jackson, PF Optimization of arylindenopyrimidines as potent adenosine A(2A)/A(1) antagonists. Bioorg Med Chem Lett20:2868-71 (2010) [PubMed] Article
Shook, BC; Rassnick, S; Chakravarty, D; Wallace, N; Ault, M; Crooke, J; Barbay, JK; Wang, A; Leonard, K; Powell, MT; Alford, V; Hall, D; Rupert, KC; Heintzelman, GR; Hansen, K; Bullington, JL; Scannevin, RH; Carroll, K; Lampron, L; Westover, L; Russell, R; Branum, S; Wells, K; Damon, S; Youells, S; Beauchamp, D; Li, X; Rhodes, K; Jackson, PF Optimization of arylindenopyrimidines as potent adenosine A(2A)/A(1) antagonists. Bioorg Med Chem Lett20:2868-71 (2010) [PubMed] Article