

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Cytochrome P450 3A4 | ||
| Ligand | BDBM50323721 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_646079 (CHEMBL1216220) | ||
| IC50 | 58500±n/a nM | ||
| Citation |  Hopkins, S; Scorneaux, B; Huang, Z; Murray, MG; Wring, S; Smitley, C; Harris, R; Erdmann, F; Fischer, G; Ribeill, Y SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro. Antimicrob Agents Chemother54:660-72 (2010) [PubMed] Article Hopkins, S; Scorneaux, B; Huang, Z; Murray, MG; Wring, S; Smitley, C; Harris, R; Erdmann, F; Fischer, G; Ribeill, Y SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro. Antimicrob Agents Chemother54:660-72 (2010) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Cytochrome P450 3A4 | |||
| Name: | Cytochrome P450 3A4 | ||
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 57349.57 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | n/a | ||
| Residue: | 503 | ||
| Sequence: |
| ||
| BDBM50323721 | |||
| n/a | |||
| Name | BDBM50323721 | ||
| Synonyms: | (3S,6S,9S,12R,15S,18S,21S,24S,27S,30S,33S)-27-(2-(dimethylamino)ethylthio)-30-ethyl-33-((1R,2R,E)-1-hydroxy-2-methylhex-4-enyl)-24-(2-hydroxy-2-methylpropyl)-6,9,18-triisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone | CHEMBL1213341 | SCY-635 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C66H120N12O13S | ||
| Mol. Mass. | 1321.797 | ||
| SMILES | CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)(C)O)N(C)C(=O)[C@H](SCCN(C)C)N(C)C1=O)C(C)C |r| | ||
| Structure | 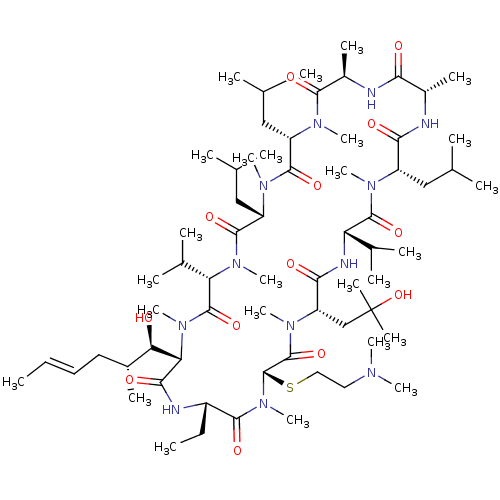
| ||