 Found 2948 hits with Last Name = 'harris' and Initial = 'r'
Found 2948 hits with Last Name = 'harris' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A,L591M]
(Human immunodeficiency virus type 1) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
| Assay Description
The inhibition assays were performed in microtiter plate wells by mixing enzyme and fluorescent peptide substrate in the presence of inhibitor compou... |
J Med Chem 49: 1379-87 (2006)
Article DOI: 10.1021/jm050943c
BindingDB Entry DOI: 10.7270/Q20C4T0V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415991

(CHEMBL1084794)Show SMILES NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C19H20N2O2S/c20-11-14-5-3-4-13-10-15(8-9-16(13)14)24(22,23)19-12-21-18-7-2-1-6-17(18)19/h1-2,6-10,12,14,21H,3-5,11,20H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415975
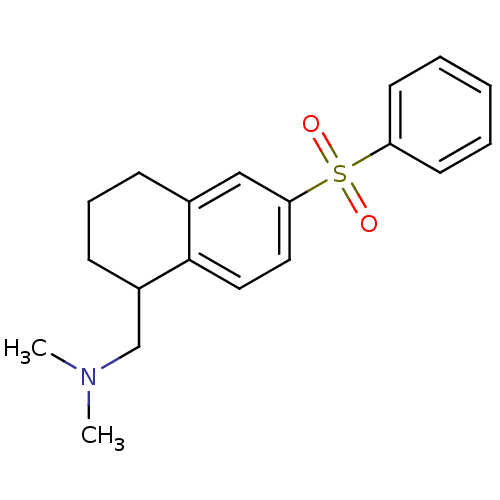
(CHEMBL1085462)Show InChI InChI=1S/C19H23NO2S/c1-20(2)14-16-8-6-7-15-13-18(11-12-19(15)16)23(21,22)17-9-4-3-5-10-17/h3-5,9-13,16H,6-8,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183152

(CHEMBL206580 | N-(4-(trifluoromethyl)benzyl)-N-iso...)Show InChI InChI=1S/C17H25F3N2/c1-13(2)11-22(16-7-9-21-10-8-16)12-14-3-5-15(6-4-14)17(18,19)20/h3-6,13,16,21H,7-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415981

(CHEMBL1086326)Show SMILES NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C17H18FNO2S/c18-14-5-2-6-15(10-14)22(20,21)16-7-8-17-12(9-16)3-1-4-13(17)11-19/h2,5-10,13H,1,3-4,11,19H2/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22000
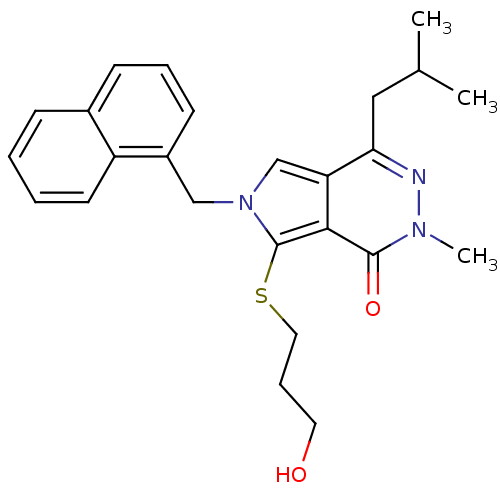
(7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...)Show SMILES CC(C)Cc1nn(C)c(=O)c2c(SCCCO)n(Cc3cccc4ccccc34)cc12 Show InChI InChI=1S/C25H29N3O2S/c1-17(2)14-22-21-16-28(15-19-10-6-9-18-8-4-5-11-20(18)19)25(31-13-7-12-29)23(21)24(30)27(3)26-22/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416028
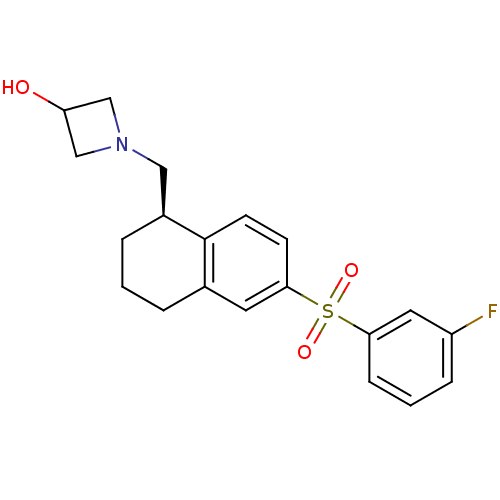
(CHEMBL1085658)Show SMILES OC1CN(C[C@@H]2CCCc3cc(ccc23)S(=O)(=O)c2cccc(F)c2)C1 |r| Show InChI InChI=1S/C20H22FNO3S/c21-16-5-2-6-18(10-16)26(24,25)19-7-8-20-14(9-19)3-1-4-15(20)11-22-12-17(23)13-22/h2,5-10,15,17,23H,1,3-4,11-13H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415993
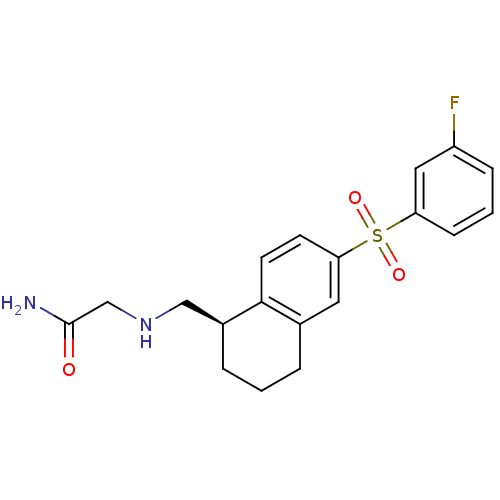
(CHEMBL1086252)Show SMILES NC(=O)CNC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H21FN2O3S/c20-15-5-2-6-16(10-15)26(24,25)17-7-8-18-13(9-17)3-1-4-14(18)11-22-12-19(21)23/h2,5-10,14,22H,1,3-4,11-12H2,(H2,21,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416007

(CHEMBL1085120)Show SMILES OCC(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H20FNO4S/c20-15-5-2-6-16(10-15)26(24,25)17-7-8-18-13(9-17)3-1-4-14(18)11-21-19(23)12-22/h2,5-10,14,22H,1,3-4,11-12H2,(H,21,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416015

(CHEMBL1086113)Show SMILES Oc1c[nH]c(NC[C@@H]2CCCc3cc(ccc23)S(=O)(=O)c2cccc(F)c2)n1 |r| Show InChI InChI=1S/C20H20FN3O3S/c21-15-5-2-6-16(10-15)28(26,27)17-7-8-18-13(9-17)3-1-4-14(18)11-22-20-23-12-19(25)24-20/h2,5-10,12,14,25H,1,3-4,11H2,(H2,22,23,24)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416006
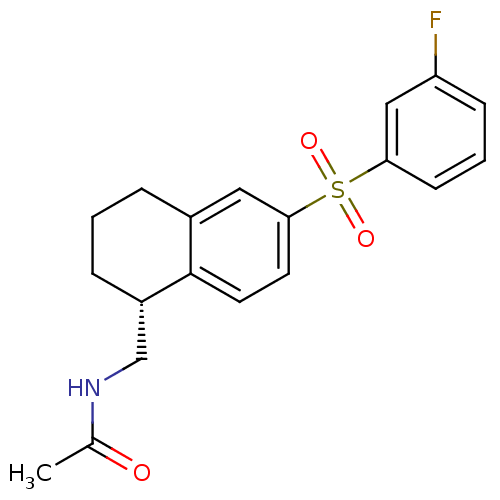
(CHEMBL1083886)Show SMILES CC(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H20FNO3S/c1-13(22)21-12-15-5-2-4-14-10-18(8-9-19(14)15)25(23,24)17-7-3-6-16(20)11-17/h3,6-11,15H,2,4-5,12H2,1H3,(H,21,22)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416012
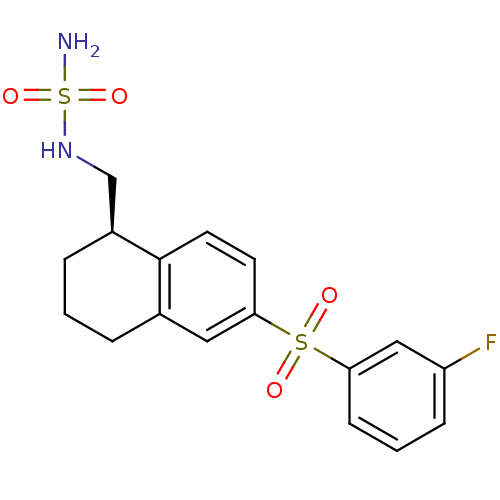
(CHEMBL1084711)Show SMILES NS(=O)(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C17H19FN2O4S2/c18-14-5-2-6-15(10-14)25(21,22)16-7-8-17-12(9-16)3-1-4-13(17)11-20-26(19,23)24/h2,5-10,13,20H,1,3-4,11H2,(H2,19,23,24)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415996

(CHEMBL1085037)Show SMILES OCCNC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H22FNO3S/c20-16-5-2-6-17(12-16)25(23,24)18-7-8-19-14(11-18)3-1-4-15(19)13-21-9-10-22/h2,5-8,11-12,15,21-22H,1,3-4,9-10,13H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416019
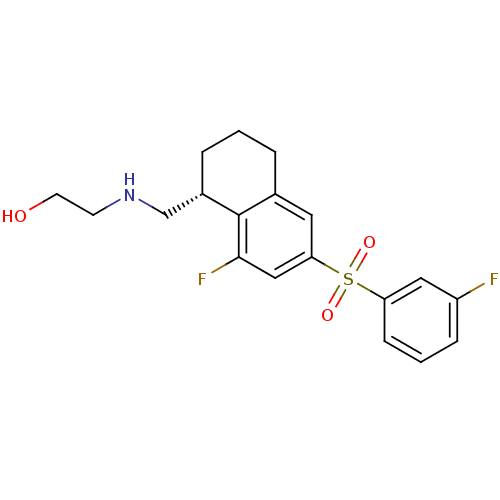
(CHEMBL1084336)Show SMILES OCCNC[C@@H]1CCCc2cc(cc(F)c12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H21F2NO3S/c20-15-5-2-6-16(10-15)26(24,25)17-9-13-3-1-4-14(12-22-7-8-23)19(13)18(21)11-17/h2,5-6,9-11,14,22-23H,1,3-4,7-8,12H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415987
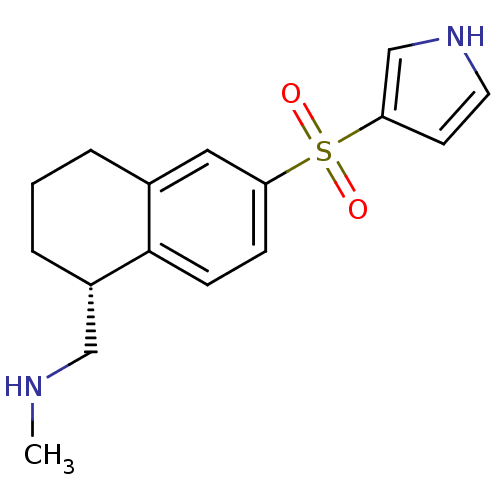
(CHEMBL1085617)Show SMILES CNC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cc[nH]c1 |r| Show InChI InChI=1S/C16H20N2O2S/c1-17-10-13-4-2-3-12-9-14(5-6-16(12)13)21(19,20)15-7-8-18-11-15/h5-9,11,13,17-18H,2-4,10H2,1H3/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415978
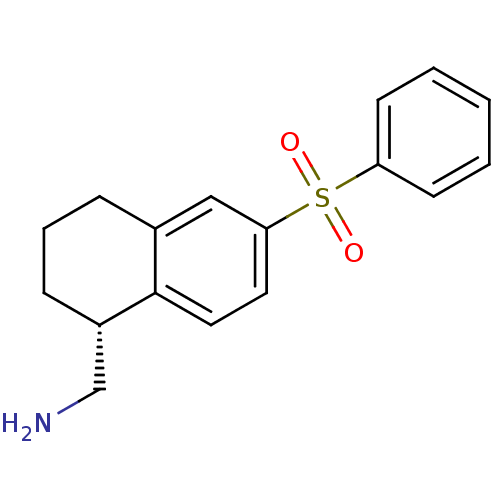
(CHEMBL1086323)Show SMILES NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C17H19NO2S/c18-12-14-6-4-5-13-11-16(9-10-17(13)14)21(19,20)15-7-2-1-3-8-15/h1-3,7-11,14H,4-6,12,18H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415972

(CHEMBL1086079)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-1-[#6]-[#6]-[#6]-c2cc(ccc-12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C18H21N3O2S/c19-18(20)21-12-14-6-4-5-13-11-16(9-10-17(13)14)24(22,23)15-7-2-1-3-8-15/h1-3,7-11,14H,4-6,12H2,(H4,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416000
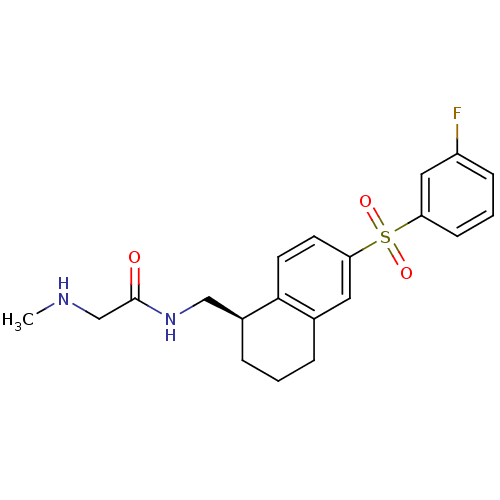
(CHEMBL1085585)Show SMILES CNCC(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C20H23FN2O3S/c1-22-13-20(24)23-12-15-5-2-4-14-10-18(8-9-19(14)15)27(25,26)17-7-3-6-16(21)11-17/h3,6-11,15,22H,2,4-5,12-13H2,1H3,(H,23,24)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416010

(CHEMBL1083781)Show SMILES CS(=O)(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C18H20FNO4S2/c1-25(21,22)20-12-14-5-2-4-13-10-17(8-9-18(13)14)26(23,24)16-7-3-6-15(19)11-16/h3,6-11,14,20H,2,4-5,12H2,1H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416029
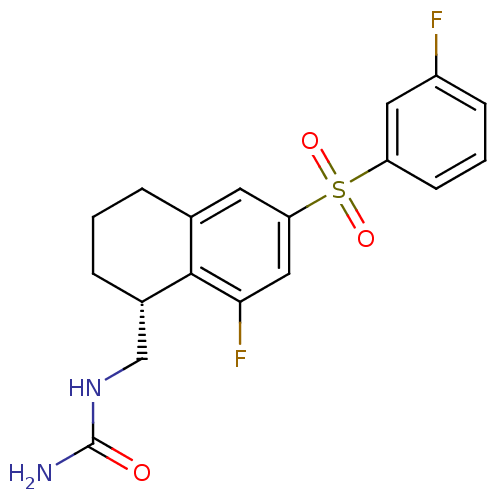
(CHEMBL1084603)Show SMILES NC(=O)NC[C@@H]1CCCc2cc(cc(F)c12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C18H18F2N2O3S/c19-13-5-2-6-14(8-13)26(24,25)15-7-11-3-1-4-12(10-22-18(21)23)17(11)16(20)9-15/h2,5-9,12H,1,3-4,10H2,(H3,21,22,23)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416003
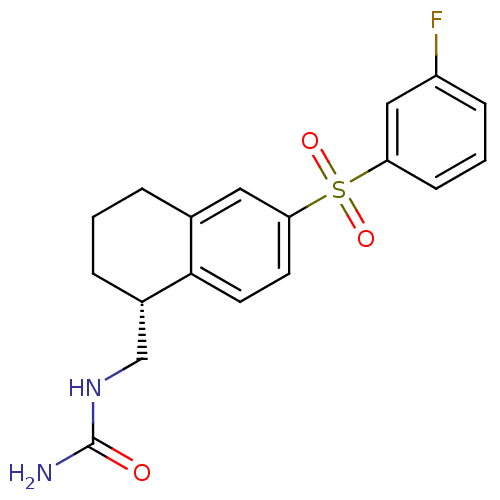
(CHEMBL1082508)Show SMILES NC(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C18H19FN2O3S/c19-14-5-2-6-15(10-14)25(23,24)16-7-8-17-12(9-16)3-1-4-13(17)11-21-18(20)22/h2,5-10,13H,1,3-4,11H2,(H3,20,21,22)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.220 | -57.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Georgia State University
| Assay Description
The inhibition assays were performed in microtiter plate wells by mixing enzyme and fluorescent peptide substrate in the presence of inhibitor compou... |
J Med Chem 49: 1379-87 (2006)
Article DOI: 10.1021/jm050943c
BindingDB Entry DOI: 10.7270/Q20C4T0V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415997
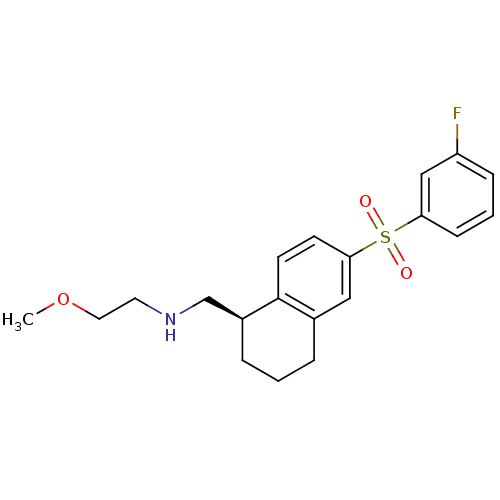
(CHEMBL1085038)Show SMILES COCCNC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C20H24FNO3S/c1-25-11-10-22-14-16-5-2-4-15-12-19(8-9-20(15)16)26(23,24)18-7-3-6-17(21)13-18/h3,6-9,12-13,16,22H,2,4-5,10-11,14H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416031
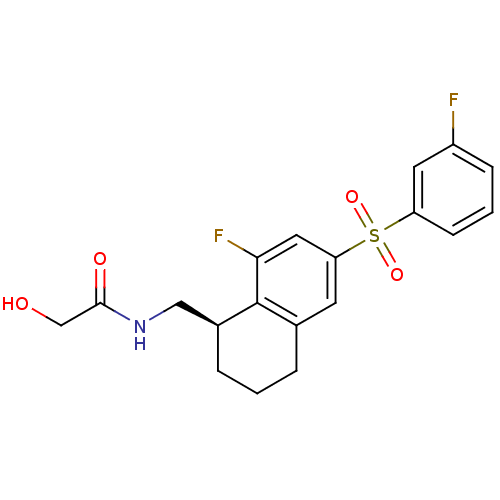
(CHEMBL1084604)Show SMILES OCC(=O)NC[C@@H]1CCCc2cc(cc(F)c12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H19F2NO4S/c20-14-5-2-6-15(8-14)27(25,26)16-7-12-3-1-4-13(10-22-18(24)11-23)19(12)17(21)9-16/h2,5-9,13,23H,1,3-4,10-11H2,(H,22,24)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415979

(CHEMBL1086324)Show SMILES NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(O)c1 |r| Show InChI InChI=1S/C17H19NO3S/c18-11-13-4-1-3-12-9-16(7-8-17(12)13)22(20,21)15-6-2-5-14(19)10-15/h2,5-10,13,19H,1,3-4,11,18H2/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183124
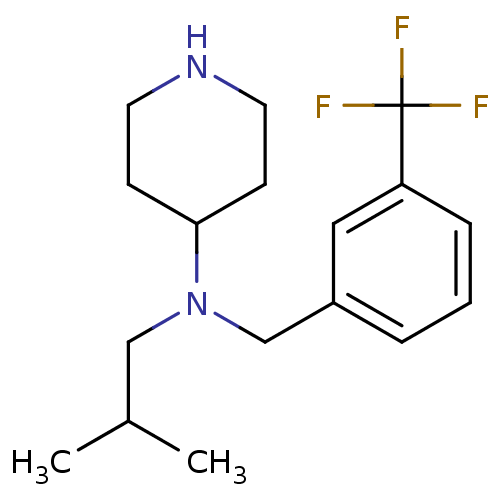
(CHEMBL381373 | N-(3-(trifluoromethyl)benzyl)-N-iso...)Show InChI InChI=1S/C17H25F3N2/c1-13(2)11-22(16-6-8-21-9-7-16)12-14-4-3-5-15(10-14)17(18,19)20/h3-5,10,13,16,21H,6-9,11-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50476647
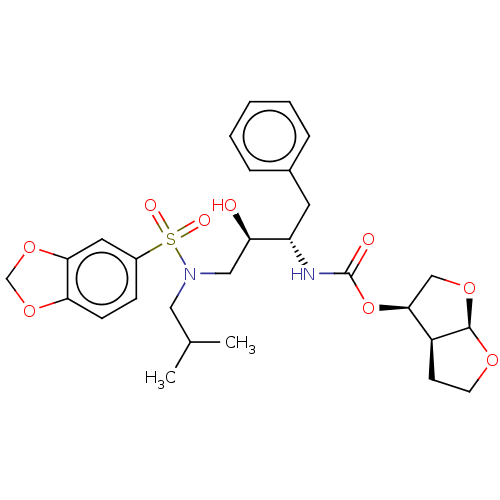
(CHEMBL178593 | GRL-98065)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C28H36N2O9S/c1-18(2)14-30(40(33,34)20-8-9-24-25(13-20)38-17-37-24)15-23(31)22(12-19-6-4-3-5-7-19)29-28(32)39-26-16-36-27-21(26)10-11-35-27/h3-9,13,18,21-23,26-27,31H,10-12,14-17H2,1-2H3,(H,29,32)/t21-,22-,23+,26-,27+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 50: 4509-15 (2007)
Article DOI: 10.1021/jm070482q
BindingDB Entry DOI: 10.7270/Q21G0Q1W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22001
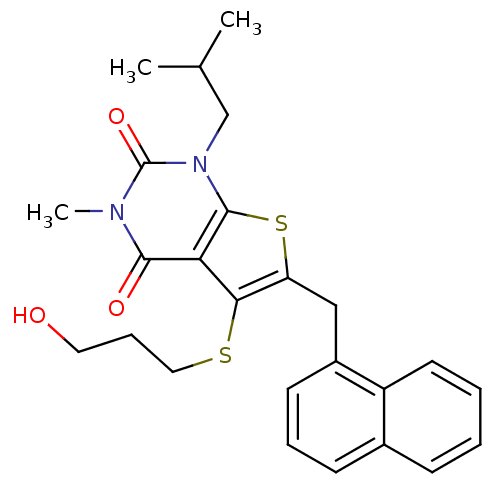
(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H28N2O3S2/c1-16(2)15-27-24-21(23(29)26(3)25(27)30)22(31-13-7-12-28)20(32-24)14-18-10-6-9-17-8-4-5-11-19(17)18/h4-6,8-11,16,28H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183122

(4-((isobutyl(piperidin-4-yl)amino)methyl)benzonitr...)Show InChI InChI=1S/C17H25N3/c1-14(2)12-20(17-7-9-19-10-8-17)13-16-5-3-15(11-18)4-6-16/h3-6,14,17,19H,7-10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183121
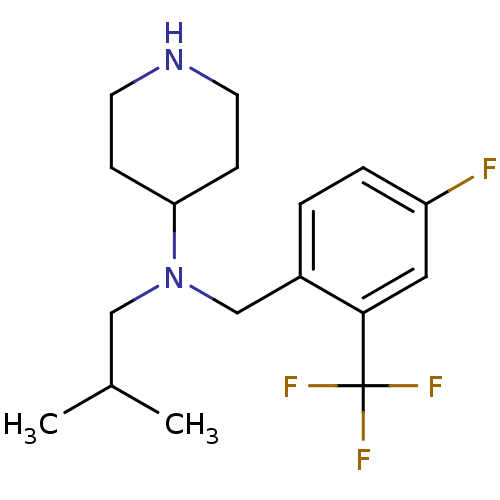
(CHEMBL441358 | N-(4-fluoro-2-(trifluoromethyl)benz...)Show InChI InChI=1S/C17H24F4N2/c1-12(2)10-23(15-5-7-22-8-6-15)11-13-3-4-14(18)9-16(13)17(19,20)21/h3-4,9,12,15,22H,5-8,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22001

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H28N2O3S2/c1-16(2)15-27-24-21(23(29)26(3)25(27)30)22(31-13-7-12-28)20(32-24)14-18-10-6-9-17-8-4-5-11-19(17)18/h4-6,8-11,16,28H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415974
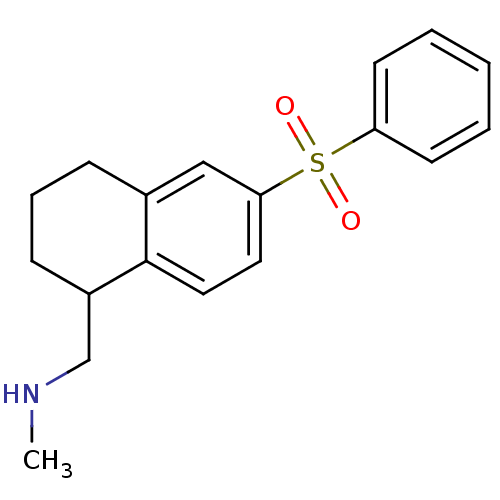
(CHEMBL1085111)Show InChI InChI=1S/C18H21NO2S/c1-19-13-15-7-5-6-14-12-17(10-11-18(14)15)22(20,21)16-8-3-2-4-9-16/h2-4,8-12,15,19H,5-7,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415999
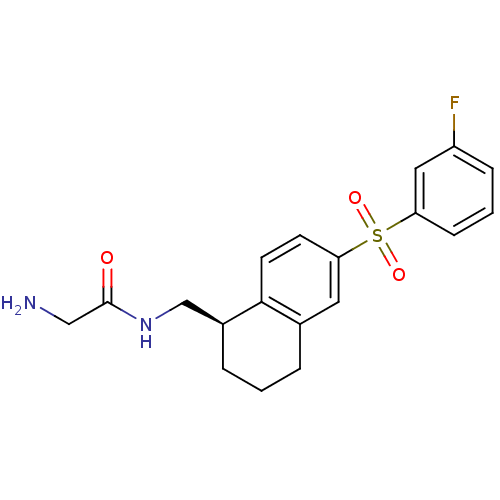
(CHEMBL1086477)Show SMILES NCC(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H21FN2O3S/c20-15-5-2-6-16(10-15)26(24,25)17-7-8-18-13(9-17)3-1-4-14(18)12-22-19(23)11-21/h2,5-10,14H,1,3-4,11-12,21H2,(H,22,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416030
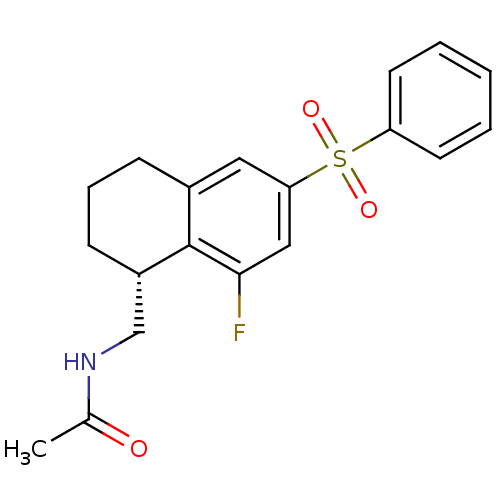
(CHEMBL1082777)Show SMILES CC(=O)NC[C@@H]1CCCc2cc(cc(F)c12)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C19H20FNO3S/c1-13(22)21-12-15-7-5-6-14-10-17(11-18(20)19(14)15)25(23,24)16-8-3-2-4-9-16/h2-4,8-11,15H,5-7,12H2,1H3,(H,21,22)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415986
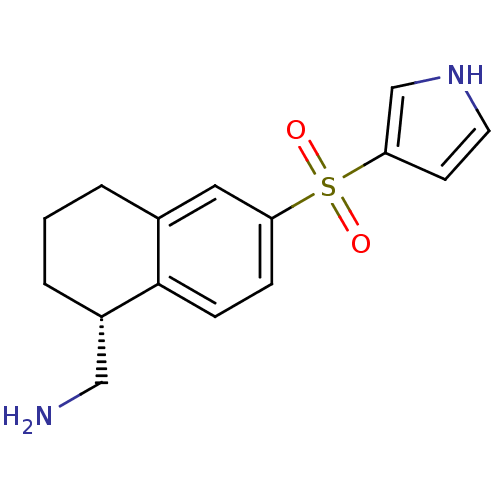
(CHEMBL1085372)Show SMILES NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cc[nH]c1 |r| Show InChI InChI=1S/C15H18N2O2S/c16-9-12-3-1-2-11-8-13(4-5-15(11)12)20(18,19)14-6-7-17-10-14/h4-8,10,12,17H,1-3,9,16H2/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21986

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2cn(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H29N3O3S/c1-17(2)14-28-21-16-27(15-19-10-6-9-18-8-4-5-11-20(18)19)24(32-13-7-12-29)22(21)23(30)26(3)25(28)31/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21986

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2cn(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H29N3O3S/c1-17(2)14-28-21-16-27(15-19-10-6-9-18-8-4-5-11-20(18)19)24(32-13-7-12-29)22(21)23(30)26(3)25(28)31/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 50: 4509-15 (2007)
Article DOI: 10.1021/jm070482q
BindingDB Entry DOI: 10.7270/Q21G0Q1W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22002
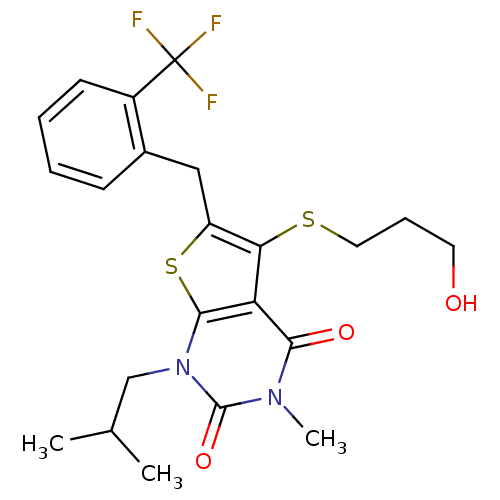
(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3ccccc3C(F)(F)F)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C22H25F3N2O3S2/c1-13(2)12-27-20-17(19(29)26(3)21(27)30)18(31-10-6-9-28)16(32-20)11-14-7-4-5-8-15(14)22(23,24)25/h4-5,7-8,13,28H,6,9-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22002

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3ccccc3C(F)(F)F)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C22H25F3N2O3S2/c1-13(2)12-27-20-17(19(29)26(3)21(27)30)18(31-10-6-9-28)16(32-20)11-14-7-4-5-8-15(14)22(23,24)25/h4-5,7-8,13,28H,6,9-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289127
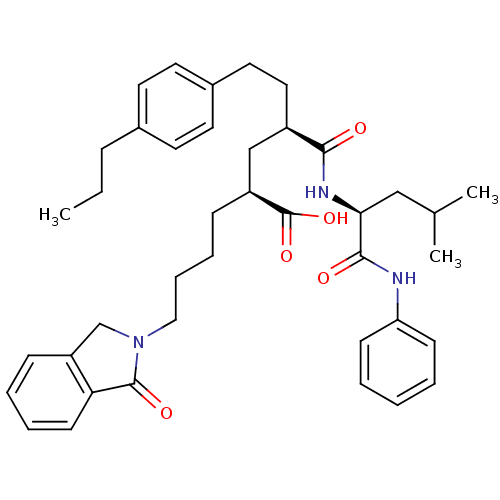
((2S,4R)-4-(3-Methyl-1-phenylcarbamoyl-butylcarbamo...)Show SMILES CCCc1ccc(CC[C@H](C[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C40H51N3O5/c1-4-12-29-18-20-30(21-19-29)22-23-31(37(44)42-36(25-28(2)3)38(45)41-34-15-6-5-7-16-34)26-32(40(47)48)13-10-11-24-43-27-33-14-8-9-17-35(33)39(43)46/h5-9,14-21,28,31-32,36H,4,10-13,22-27H2,1-3H3,(H,41,45)(H,42,44)(H,47,48)/t31-,32+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183126

(CHEMBL207374 | N-(2,4-dimethylbenzyl)-N-isobutylpi...)Show InChI InChI=1S/C18H30N2/c1-14(2)12-20(18-7-9-19-10-8-18)13-17-6-5-15(3)11-16(17)4/h5-6,11,14,18-19H,7-10,12-13H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50415980
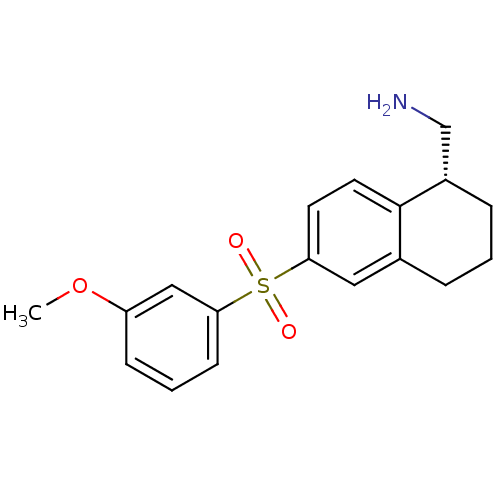
(CHEMBL1086325)Show SMILES COc1cccc(c1)S(=O)(=O)c1ccc2[C@H](CN)CCCc2c1 |r| Show InChI InChI=1S/C18H21NO3S/c1-22-15-6-3-7-16(11-15)23(20,21)17-8-9-18-13(10-17)4-2-5-14(18)12-19/h3,6-11,14H,2,4-5,12,19H2,1H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416001

(CHEMBL1084071)Show SMILES CN(C)CC(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C21H25FN2O3S/c1-24(2)14-21(25)23-13-16-6-3-5-15-11-19(9-10-20(15)16)28(26,27)18-8-4-7-17(22)12-18/h4,7-12,16H,3,5-6,13-14H2,1-2H3,(H,23,25)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416020
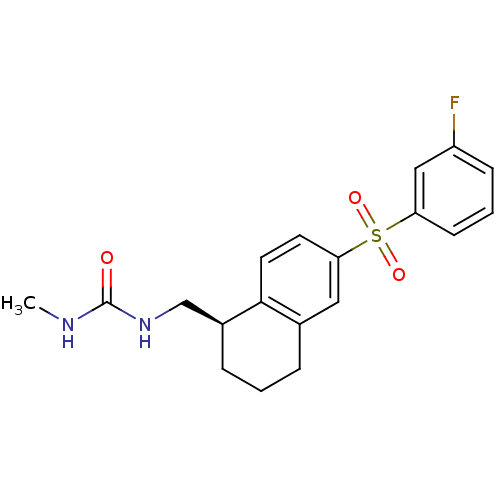
(CHEMBL1082509)Show SMILES CNC(=O)NC[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C19H21FN2O3S/c1-21-19(23)22-12-14-5-2-4-13-10-17(8-9-18(13)14)26(24,25)16-7-3-6-15(20)11-16/h3,6-11,14H,2,4-5,12H2,1H3,(H2,21,22,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416024

(CHEMBL1083782)Show SMILES CN(C[C@@H]1CCCc2cc(ccc12)S(=O)(=O)c1cccc(F)c1)S(C)(=O)=O |r| Show InChI InChI=1S/C19H22FNO4S2/c1-21(26(2,22)23)13-15-6-3-5-14-11-18(9-10-19(14)15)27(24,25)17-8-4-7-16(20)12-17/h4,7-12,15H,3,5-6,13H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD form human recombinant 5HT6 receptor |
Bioorg Med Chem Lett 20: 3436-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.110
BindingDB Entry DOI: 10.7270/Q2GX4CT3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183177

(4-((4-fluoro-2-(trifluoromethyl)benzyl)(piperidin-...)Show InChI InChI=1S/C17H21F4N3/c18-14-4-3-13(16(11-14)17(19,20)21)12-24(10-2-1-7-22)15-5-8-23-9-6-15/h3-4,11,15,23H,1-2,5-6,8-10,12H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183141

(3-((4-fluoro-2-(trifluoromethyl)benzyl)(piperidin-...)Show InChI InChI=1S/C16H19F4N3/c17-13-3-2-12(15(10-13)16(18,19)20)11-23(9-1-6-21)14-4-7-22-8-5-14/h2-3,10,14,22H,1,4-5,7-9,11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183154

(CHEMBL207067 | CHEMBL207214 | N-(4-fluoro-2-(trifl...)Show InChI InChI=1S/C17H22F4N2/c18-14-4-3-13(16(9-14)17(19,20)21)11-23(10-12-1-2-12)15-5-7-22-8-6-15/h3-4,9,12,15,22H,1-2,5-8,10-11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data