 Found 33 hits with Last Name = 'murray' and Initial = 'mg'
Found 33 hits with Last Name = 'murray' and Initial = 'mg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50323721
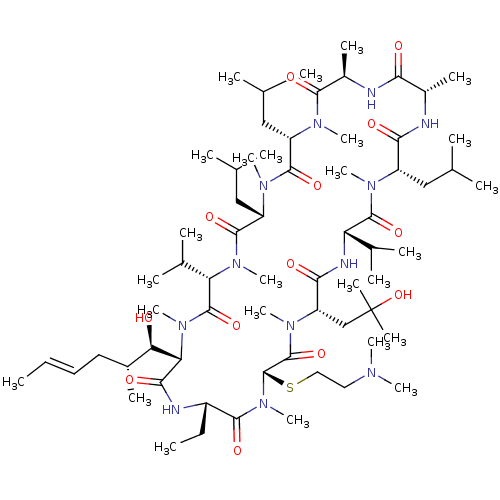
((3S,6S,9S,12R,15S,18S,21S,24S,27S,30S,33S)-27-(2-(...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)(C)O)N(C)C(=O)[C@H](SCCN(C)C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H120N12O13S/c1-27-29-30-42(13)53(79)52-57(83)69-45(28-2)59(85)78(26)65(92-32-31-71(18)19)64(90)75(23)49(36-66(16,17)91)56(82)70-50(40(9)10)62(88)72(20)46(33-37(3)4)55(81)67-43(14)54(80)68-44(15)58(84)73(21)47(34-38(5)6)60(86)74(22)48(35-39(7)8)61(87)76(24)51(41(11)12)63(89)77(52)25/h27,29,37-53,65,79,91H,28,30-36H2,1-26H3,(H,67,81)(H,68,80)(H,69,83)(H,70,82)/b29-27+/t42-,43+,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-,65+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of calcineurin phosphatase activity of CyPA |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104946
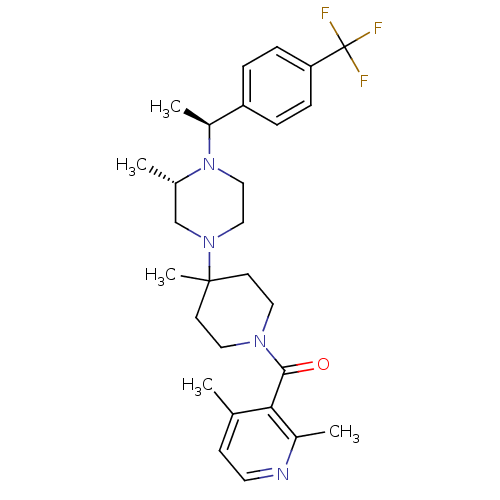
((2,4-Dimethyl-pyridin-3-yl)-(4-methyl-4-{(S)-3-met...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ccnc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O/c1-19-10-13-32-21(3)25(19)26(36)33-14-11-27(5,12-15-33)34-16-17-35(20(2)18-34)22(4)23-6-8-24(9-7-23)28(29,30)31/h6-10,13,20,22H,11-12,14-18H2,1-5H3/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of calcineurin phosphatase activity of CyPA |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104950
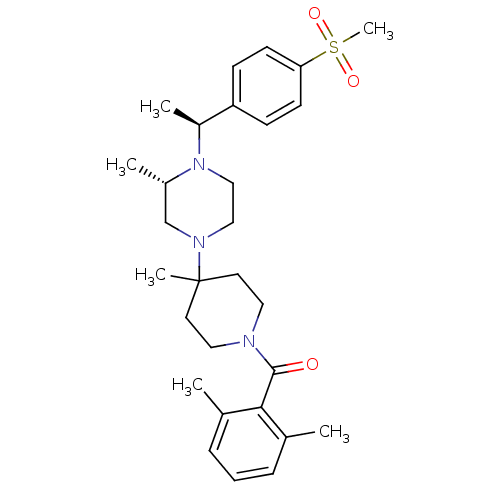
((2,6-Dimethyl-phenyl)-(4-{(S)-4-[(S)-1-(4-methanes...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cccc1C)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C29H41N3O3S/c1-21-8-7-9-22(2)27(21)28(33)30-16-14-29(5,15-17-30)31-18-19-32(23(3)20-31)24(4)25-10-12-26(13-11-25)36(6,34)35/h7-13,23-24H,14-20H2,1-6H3/t23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104953

((2-Amino-6-chloro-phenyl)-(4-{(S)-4-[(S)-1-(4-iodo...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(N)cccc1Cl)c1ccc(I)cc1 Show InChI InChI=1S/C26H34ClIN4O/c1-18-17-31(15-16-32(18)19(2)20-7-9-21(28)10-8-20)26(3)11-13-30(14-12-26)25(33)24-22(27)5-4-6-23(24)29/h4-10,18-19H,11-17,29H2,1-3H3/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104952

((2,6-Dimethyl-phenyl)-(4-{(S)-4-[(S)-1-(4-iodo-phe...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cccc1C)c1ccc(I)cc1 Show InChI InChI=1S/C28H38IN3O/c1-20-7-6-8-21(2)26(20)27(33)30-15-13-28(5,14-16-30)31-17-18-32(22(3)19-31)23(4)24-9-11-25(29)12-10-24/h6-12,22-23H,13-19H2,1-5H3/t22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104944
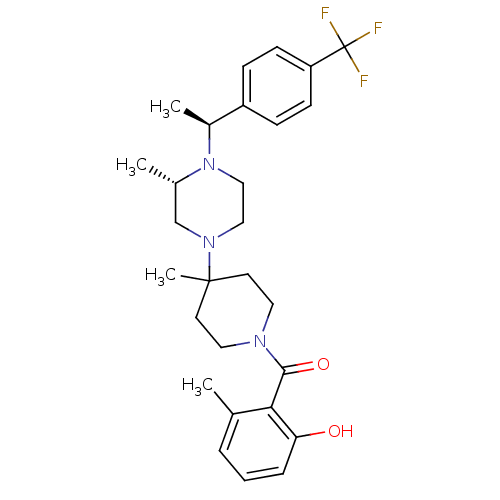
((2-Hydroxy-6-methyl-phenyl)-(4-methyl-4-{(S)-3-met...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cccc1O)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H36F3N3O2/c1-19-6-5-7-24(35)25(19)26(36)32-14-12-27(4,13-15-32)33-16-17-34(20(2)18-33)21(3)22-8-10-23(11-9-22)28(29,30)31/h5-11,20-21,35H,12-18H2,1-4H3/t20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104955

((2-Amino-6-chloro-phenyl)-(4-methyl-4-{(S)-3-methy...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(N)cccc1Cl)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C27H34ClF3N4O/c1-18-17-34(15-16-35(18)19(2)20-7-9-21(10-8-20)27(29,30)31)26(3)11-13-33(14-12-26)25(36)24-22(28)5-4-6-23(24)32/h4-10,18-19H,11-17,32H2,1-3H3/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104958

((2-Amino-6-chloro-phenyl)-(4-{(S)-4-[(S)-1-(4-meth...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(N)cccc1Cl)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C27H37ClN4O3S/c1-19-18-31(16-17-32(19)20(2)21-8-10-22(11-9-21)36(4,34)35)27(3)12-14-30(15-13-27)26(33)25-23(28)6-5-7-24(25)29/h5-11,19-20H,12-18,29H2,1-4H3/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104954
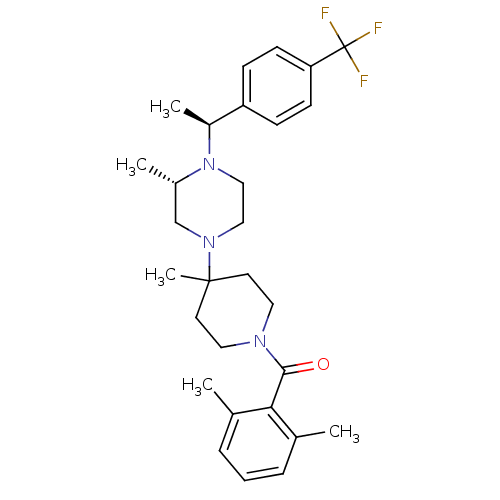
((2,6-Dimethyl-phenyl)-(4-methyl-4-{(S)-3-methyl-4-...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cccc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H38F3N3O/c1-20-7-6-8-21(2)26(20)27(36)33-15-13-28(5,14-16-33)34-17-18-35(22(3)19-34)23(4)24-9-11-25(12-10-24)29(30,31)32/h6-12,22-23H,13-19H2,1-5H3/t22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104956
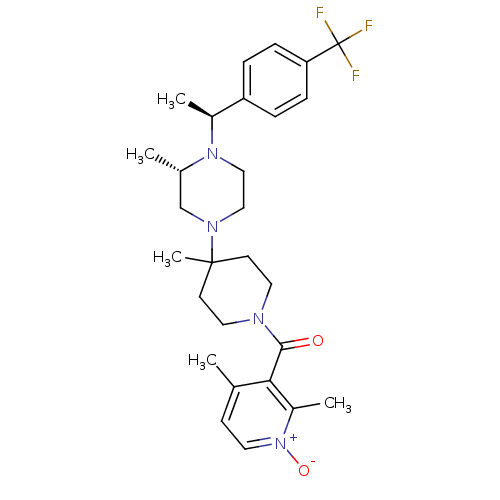
((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-methyl-4-{(S)...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cc[n+]([O-])c1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O2/c1-19-10-13-35(37)22(4)25(19)26(36)32-14-11-27(5,12-15-32)33-16-17-34(20(2)18-33)21(3)23-6-8-24(9-7-23)28(29,30)31/h6-10,13,20-21H,11-12,14-18H2,1-5H3/t20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104949
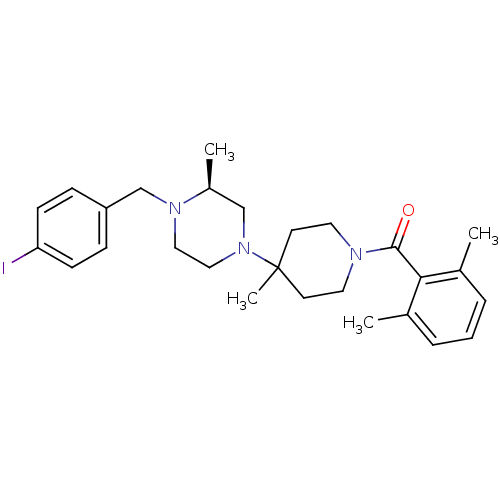
((2,6-Dimethyl-phenyl)-{4-[(S)-4-(4-iodo-benzyl)-3-...)Show SMILES C[C@H]1CN(CCN1Cc1ccc(I)cc1)C1(C)CCN(CC1)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C27H36IN3O/c1-20-6-5-7-21(2)25(20)26(32)29-14-12-27(4,13-15-29)31-17-16-30(22(3)18-31)19-23-8-10-24(28)11-9-23/h5-11,22H,12-19H2,1-4H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104951

((2-Hydroxy-6-methyl-phenyl)-(4-{(S)-4-[(S)-1-(4-io...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cccc1O)c1ccc(I)cc1 Show InChI InChI=1S/C27H36IN3O2/c1-19-6-5-7-24(32)25(19)26(33)29-14-12-27(4,13-15-29)30-16-17-31(20(2)18-30)21(3)22-8-10-23(28)11-9-22/h5-11,20-21,32H,12-18H2,1-4H3/t20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104959
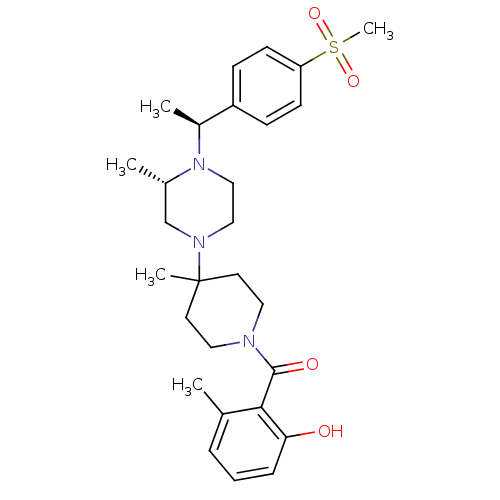
((2-Hydroxy-6-methyl-phenyl)-(4-{(S)-4-[(S)-1-(4-me...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cccc1O)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C28H39N3O4S/c1-20-7-6-8-25(32)26(20)27(33)29-15-13-28(4,14-16-29)30-17-18-31(21(2)19-30)22(3)23-9-11-24(12-10-23)36(5,34)35/h6-12,21-22,32H,13-19H2,1-5H3/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104957
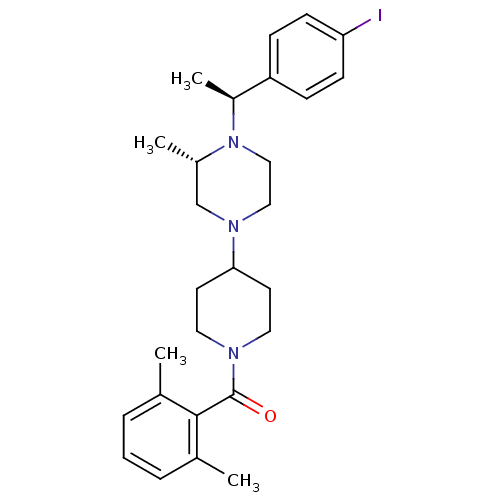
((2,6-Dimethyl-phenyl)-(4-{(S)-4-[(S)-1-(4-iodo-phe...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1CCN(CC1)C(=O)c1c(C)cccc1C)c1ccc(I)cc1 Show InChI InChI=1S/C27H36IN3O/c1-19-6-5-7-20(2)26(19)27(32)29-14-12-25(13-15-29)30-16-17-31(21(3)18-30)22(4)23-8-10-24(28)11-9-23/h5-11,21-22,25H,12-18H2,1-4H3/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104947
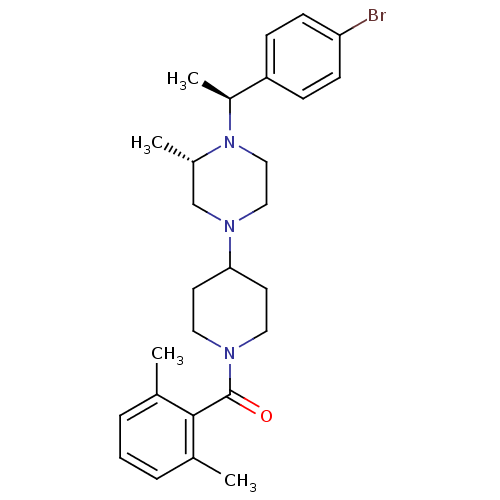
((4-{4-[1-(4-Bromo-phenyl)-ethyl]-3-methyl-piperazi...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1CCN(CC1)C(=O)c1c(C)cccc1C)c1ccc(Br)cc1 Show InChI InChI=1S/C27H36BrN3O/c1-19-6-5-7-20(2)26(19)27(32)29-14-12-25(13-15-29)30-16-17-31(21(3)18-30)22(4)23-8-10-24(28)11-9-23/h5-11,21-22,25H,12-18H2,1-4H3/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104961
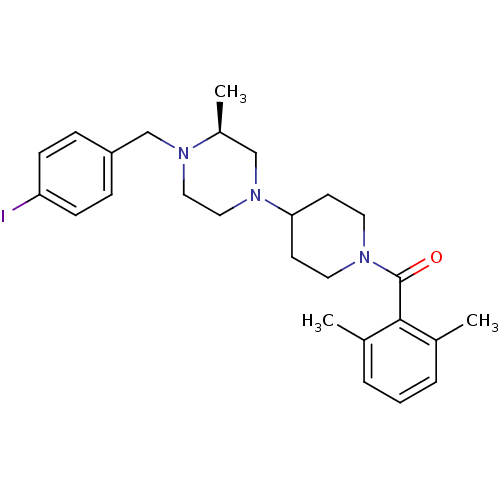
((2,6-Dimethyl-phenyl)-{4-[(S)-4-(4-iodo-benzyl)-3-...)Show SMILES C[C@H]1CN(CCN1Cc1ccc(I)cc1)C1CCN(CC1)C(=O)c1c(C)cccc1C Show InChI InChI=1S/C26H34IN3O/c1-19-5-4-6-20(2)25(19)26(31)28-13-11-24(12-14-28)30-16-15-29(21(3)17-30)18-22-7-9-23(27)10-8-22/h4-10,21,24H,11-18H2,1-3H3/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104960
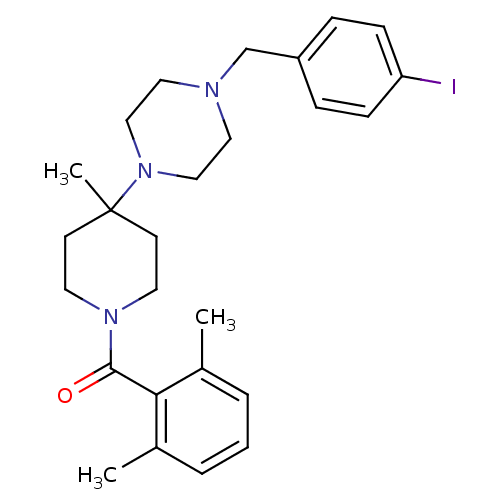
((2,6-Dimethyl-phenyl)-{4-[4-(4-iodo-benzyl)-pipera...)Show SMILES Cc1cccc(C)c1C(=O)N1CCC(C)(CC1)N1CCN(Cc2ccc(I)cc2)CC1 Show InChI InChI=1S/C26H34IN3O/c1-20-5-4-6-21(2)24(20)25(31)29-13-11-26(3,12-14-29)30-17-15-28(16-18-30)19-22-7-9-23(27)10-8-22/h4-10H,11-19H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50104956

((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-methyl-4-{(S)...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cc[n+]([O-])c1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O2/c1-19-10-13-35(37)22(4)25(19)26(36)32-14-11-27(5,12-15-32)33-16-17-34(20(2)18-33)21(3)23-6-8-24(9-7-23)28(29,30)31/h6-10,13,20-21H,11-12,14-18H2,1-5H3/t20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor M2 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104945
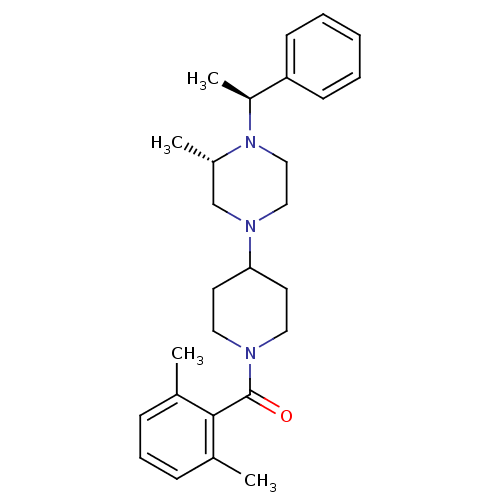
((2,6-Dimethyl-phenyl)-{4-[3-methyl-4-(1-phenyl-eth...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1CCN(CC1)C(=O)c1c(C)cccc1C)c1ccccc1 Show InChI InChI=1S/C27H37N3O/c1-20-9-8-10-21(2)26(20)27(31)28-15-13-25(14-16-28)29-17-18-30(22(3)19-29)23(4)24-11-6-5-7-12-24/h5-12,22-23,25H,13-19H2,1-4H3/t22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104948
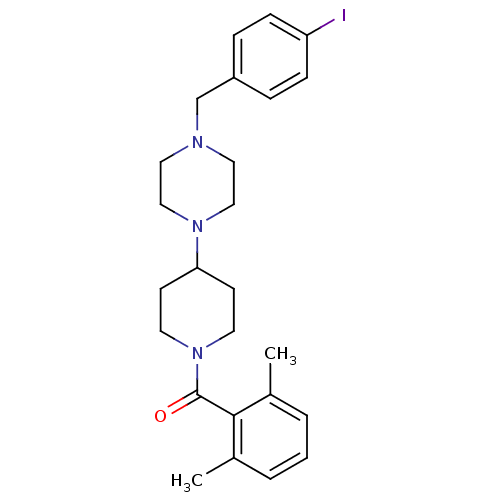
((2,6-Dimethyl-phenyl)-{4-[4-(4-iodo-benzyl)-pipera...)Show SMILES Cc1cccc(C)c1C(=O)N1CCC(CC1)N1CCN(Cc2ccc(I)cc2)CC1 Show InChI InChI=1S/C25H32IN3O/c1-19-4-3-5-20(2)24(19)25(30)29-12-10-23(11-13-29)28-16-14-27(15-17-28)18-21-6-8-22(26)9-7-21/h3-9,23H,10-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50104946

((2,4-Dimethyl-pyridin-3-yl)-(4-methyl-4-{(S)-3-met...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ccnc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O/c1-19-10-13-32-21(3)25(19)26(36)33-14-11-27(5,12-15-33)34-16-17-35(20(2)18-34)22(4)23-6-8-24(9-7-23)28(29,30)31/h6-10,13,20,22H,11-12,14-18H2,1-5H3/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor M2 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104947

((4-{4-[1-(4-Bromo-phenyl)-ethyl]-3-methyl-piperazi...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1CCN(CC1)C(=O)c1c(C)cccc1C)c1ccc(Br)cc1 Show InChI InChI=1S/C27H36BrN3O/c1-19-6-5-7-20(2)26(19)27(32)29-14-12-25(13-15-29)30-16-17-31(21(3)18-30)22(4)23-8-10-24(28)11-9-23/h5-11,21-22,25H,12-18H2,1-4H3/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against C-C chemokine receptor type 5 |
J Med Chem 44: 3343-6 (2001)
BindingDB Entry DOI: 10.7270/Q23X85X2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 after 30 mins |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 after 30 mins |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50323721

((3S,6S,9S,12R,15S,18S,21S,24S,27S,30S,33S)-27-(2-(...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)(C)O)N(C)C(=O)[C@H](SCCN(C)C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H120N12O13S/c1-27-29-30-42(13)53(79)52-57(83)69-45(28-2)59(85)78(26)65(92-32-31-71(18)19)64(90)75(23)49(36-66(16,17)91)56(82)70-50(40(9)10)62(88)72(20)46(33-37(3)4)55(81)67-43(14)54(80)68-44(15)58(84)73(21)47(34-38(5)6)60(86)74(22)48(35-39(7)8)61(87)76(24)51(41(11)12)63(89)77(52)25/h27,29,37-53,65,79,91H,28,30-36H2,1-26H3,(H,67,81)(H,68,80)(H,69,83)(H,70,82)/b29-27+/t42-,43+,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-,65+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 after 30 mins |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50323721

((3S,6S,9S,12R,15S,18S,21S,24S,27S,30S,33S)-27-(2-(...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)(C)O)N(C)C(=O)[C@H](SCCN(C)C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H120N12O13S/c1-27-29-30-42(13)53(79)52-57(83)69-45(28-2)59(85)78(26)65(92-32-31-71(18)19)64(90)75(23)49(36-66(16,17)91)56(82)70-50(40(9)10)62(88)72(20)46(33-37(3)4)55(81)67-43(14)54(80)68-44(15)58(84)73(21)47(34-38(5)6)60(86)74(22)48(35-39(7)8)61(87)76(24)51(41(11)12)63(89)77(52)25/h27,29,37-53,65,79,91H,28,30-36H2,1-26H3,(H,67,81)(H,68,80)(H,69,83)(H,70,82)/b29-27+/t42-,43+,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-,65+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 after 30 mins |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 after 30 mins |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323721

((3S,6S,9S,12R,15S,18S,21S,24S,27S,30S,33S)-27-(2-(...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)(C)O)N(C)C(=O)[C@H](SCCN(C)C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H120N12O13S/c1-27-29-30-42(13)53(79)52-57(83)69-45(28-2)59(85)78(26)65(92-32-31-71(18)19)64(90)75(23)49(36-66(16,17)91)56(82)70-50(40(9)10)62(88)72(20)46(33-37(3)4)55(81)67-43(14)54(80)68-44(15)58(84)73(21)47(34-38(5)6)60(86)74(22)48(35-39(7)8)61(87)76(24)51(41(11)12)63(89)77(52)25/h27,29,37-53,65,79,91H,28,30-36H2,1-26H3,(H,67,81)(H,68,80)(H,69,83)(H,70,82)/b29-27+/t42-,43+,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-,65+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 after 30 mins |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50323721

((3S,6S,9S,12R,15S,18S,21S,24S,27S,30S,33S)-27-(2-(...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)(C)O)N(C)C(=O)[C@H](SCCN(C)C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H120N12O13S/c1-27-29-30-42(13)53(79)52-57(83)69-45(28-2)59(85)78(26)65(92-32-31-71(18)19)64(90)75(23)49(36-66(16,17)91)56(82)70-50(40(9)10)62(88)72(20)46(33-37(3)4)55(81)67-43(14)54(80)68-44(15)58(84)73(21)47(34-38(5)6)60(86)74(22)48(35-39(7)8)61(87)76(24)51(41(11)12)63(89)77(52)25/h27,29,37-53,65,79,91H,28,30-36H2,1-26H3,(H,67,81)(H,68,80)(H,69,83)(H,70,82)/b29-27+/t42-,43+,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-,65+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 after 30 mins |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 after 30 mins |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 after 30 mins |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50323721

((3S,6S,9S,12R,15S,18S,21S,24S,27S,30S,33S)-27-(2-(...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)(C)O)N(C)C(=O)[C@H](SCCN(C)C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C66H120N12O13S/c1-27-29-30-42(13)53(79)52-57(83)69-45(28-2)59(85)78(26)65(92-32-31-71(18)19)64(90)75(23)49(36-66(16,17)91)56(82)70-50(40(9)10)62(88)72(20)46(33-37(3)4)55(81)67-43(14)54(80)68-44(15)58(84)73(21)47(34-38(5)6)60(86)74(22)48(35-39(7)8)61(87)76(24)51(41(11)12)63(89)77(52)25/h27,29,37-53,65,79,91H,28,30-36H2,1-26H3,(H,67,81)(H,68,80)(H,69,83)(H,70,82)/b29-27+/t42-,43+,44-,45+,46+,47+,48+,49+,50+,51+,52+,53-,65+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 after 30 mins |
Antimicrob Agents Chemother 54: 660-72 (2010)
Article DOI: 10.1128/AAC.00660-09
BindingDB Entry DOI: 10.7270/Q2V40VDG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data