| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2C9 |
|---|
| Ligand | BDBM50327384 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_664441 (CHEMBL1259707) |
|---|
| IC50 | 43120±n/a nM |
|---|
| Citation |  Morriello, GJ; Chicchi, G; Johnson, T; Mills, SG; Demartino, J; Kurtz, M; Tsao, KL; Zheng, S; Tong, X; Carlson, E; Townson, K; Wheeldon, A; Boyce, S; Collinson, N; Rupniak, N; Devita, RJ Fused tricyclic pyrrolizinones that exhibit pseudo-irreversible blockade of the NK1 receptor. Bioorg Med Chem Lett20:5925-32 (2010) [PubMed] Article Morriello, GJ; Chicchi, G; Johnson, T; Mills, SG; Demartino, J; Kurtz, M; Tsao, KL; Zheng, S; Tong, X; Carlson, E; Townson, K; Wheeldon, A; Boyce, S; Collinson, N; Rupniak, N; Devita, RJ Fused tricyclic pyrrolizinones that exhibit pseudo-irreversible blockade of the NK1 receptor. Bioorg Med Chem Lett20:5925-32 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2C9 |
|---|
| Name: | Cytochrome P450 2C9 |
|---|
| Synonyms: | (R)-limonene 6-monooxygenase | (S)-limonene 6-monooxygenase | CP2C9_HUMAN | CYP2C10 | CYP2C9 | CYPIIC9 | Cytochrome P450 2C9 (CYP2C9 ) | Cytochrome P450 2C9 (CYP2C9) | P-450MP | P450 MP-4/MP-8 | P450 PB-1 | S-mephenytoin 4-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 55636.33 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11712 |
|---|
| Residue: | 490 |
|---|
| Sequence: | MDSLVVLVLCLSCLLLLSLWRQSSGRGKLPPGPTPLPVIGNILQIGIKDISKSLTNLSKV
YGPVFTLYFGLKPIVVLHGYEAVKEALIDLGEEFSGRGIFPLAERANRGFGIVFSNGKKW
KEIRRFSLMTLRNFGMGKRSIEDRVQEEARCLVEELRKTKASPCDPTFILGCAPCNVICS
IIFHKRFDYKDQQFLNLMEKLNENIKILSSPWIQICNNFSPIIDYFPGTHNKLLKNVAFM
KSYILEKVKEHQESMDMNNPQDFIDCFLMKMEKEKHNQPSEFTIESLENTAVDLFGAGTE
TTSTTLRYALLLLLKHPEVTAKVQEEIERVIGRNRSPCMQDRSHMPYTDAVVHEVQRYID
LLPTSLPHAVTCDIKFRNYLIPKGTTILISLTSVLHDNKEFPNPEMFDPHHFLDEGGNFK
KSKYFMPFSAGKRICVGEALAGMELFLFLTSILQNFNLKSLVDPKNLDTTPVVNGFASVP
PFYQLCFIPV
|
|
|
|---|
| BDBM50327384 |
|---|
| n/a |
|---|
| Name | BDBM50327384 |
|---|
| Synonyms: | (1S,2R,5aS,7S,8aR,8bS)-2-((R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)-1-(4-fluorophenyl)-7-hydroxy-7-methyloctahydrocyclopenta[a]pyrrolizin-5(5aH)-one | CHEMBL1257706 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H26F7NO3 |
|---|
| Mol. Mass. | 545.4891 |
|---|
| SMILES | C[C@@H](O[C@H]1CN2[C@@H]([C@@H]3C[C@](C)(O)C[C@@H]3C2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| |
|---|
| Structure | 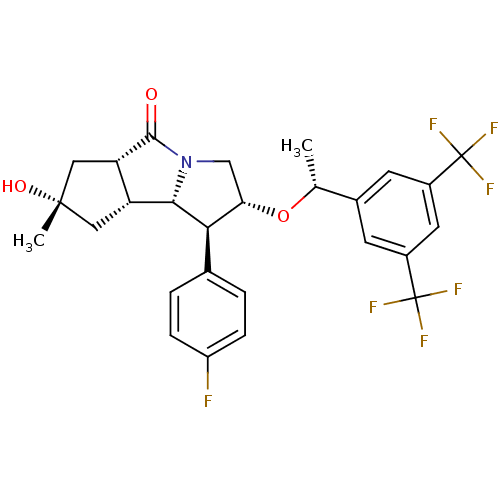
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Morriello, GJ; Chicchi, G; Johnson, T; Mills, SG; Demartino, J; Kurtz, M; Tsao, KL; Zheng, S; Tong, X; Carlson, E; Townson, K; Wheeldon, A; Boyce, S; Collinson, N; Rupniak, N; Devita, RJ Fused tricyclic pyrrolizinones that exhibit pseudo-irreversible blockade of the NK1 receptor. Bioorg Med Chem Lett20:5925-32 (2010) [PubMed] Article
Morriello, GJ; Chicchi, G; Johnson, T; Mills, SG; Demartino, J; Kurtz, M; Tsao, KL; Zheng, S; Tong, X; Carlson, E; Townson, K; Wheeldon, A; Boyce, S; Collinson, N; Rupniak, N; Devita, RJ Fused tricyclic pyrrolizinones that exhibit pseudo-irreversible blockade of the NK1 receptor. Bioorg Med Chem Lett20:5925-32 (2010) [PubMed] Article