 Found 4097 hits with Last Name = 'devita' and Initial = 'rj'
Found 4097 hits with Last Name = 'devita' and Initial = 'rj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human NK3 receptor |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human NK2 receptor |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021345
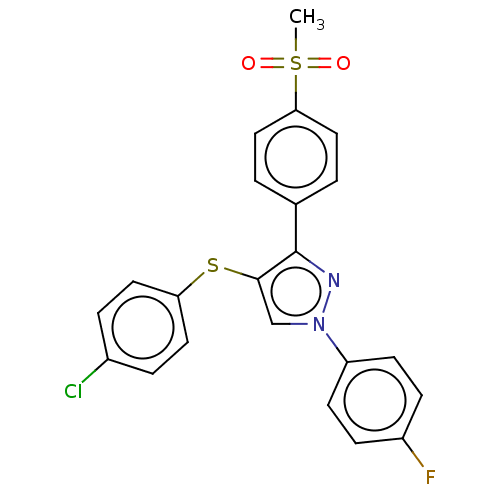
(CHEMBL3287928)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nn(cc1Sc1ccc(Cl)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H16ClFN2O2S2/c1-30(27,28)20-12-2-15(3-13-20)22-21(29-19-10-4-16(23)5-11-19)14-26(25-22)18-8-6-17(24)7-9-18/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021331
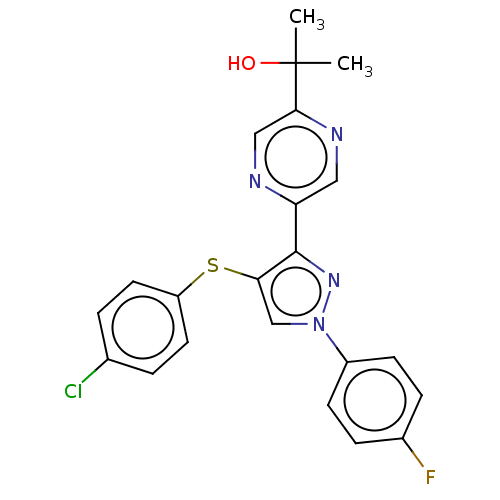
(CHEMBL3287930)Show SMILES CC(C)(O)c1cnc(cn1)-c1nn(cc1Sc1ccc(Cl)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H18ClFN4OS/c1-22(2,29)20-12-25-18(11-26-20)21-19(30-17-9-3-14(23)4-10-17)13-28(27-21)16-7-5-15(24)6-8-16/h3-13,29H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50350538
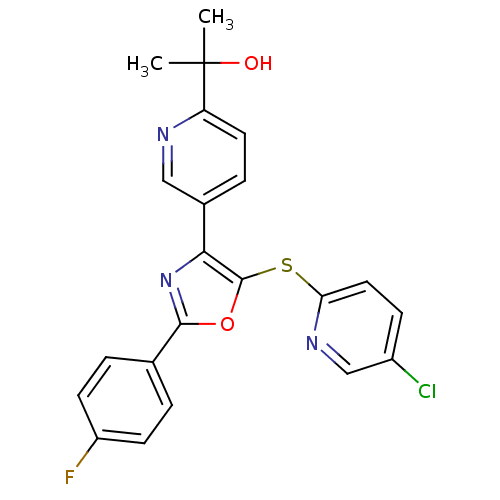
(CHEMBL1812717)Show SMILES CC(C)(O)c1ccc(cn1)-c1nc(oc1Sc1ccc(Cl)cn1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H17ClFN3O2S/c1-22(2,28)17-9-5-14(11-25-17)19-21(30-18-10-6-15(23)12-26-18)29-20(27-19)13-3-7-16(24)8-4-13/h3-12,28H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021346
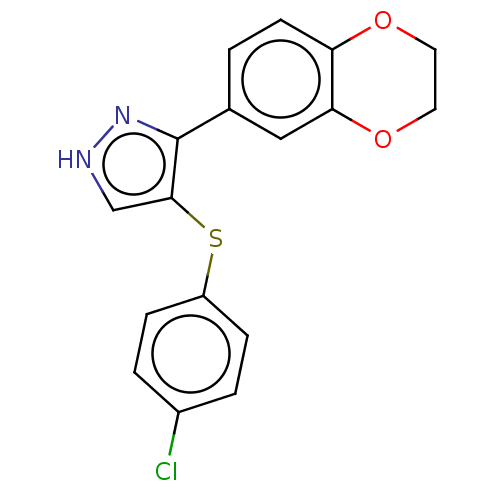
(CHEMBL3287926)Show InChI InChI=1S/C17H13ClN2O2S/c18-12-2-4-13(5-3-12)23-16-10-19-20-17(16)11-1-6-14-15(9-11)22-8-7-21-14/h1-6,9-10H,7-8H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021329
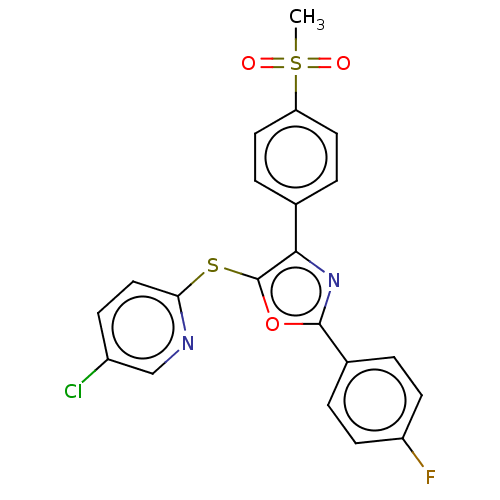
(CHEMBL3287932)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(oc1Sc1ccc(Cl)cn1)-c1ccc(F)cc1 Show InChI InChI=1S/C21H14ClFN2O3S2/c1-30(26,27)17-9-4-13(5-10-17)19-21(29-18-11-6-15(22)12-24-18)28-20(25-19)14-2-7-16(23)8-3-14/h2-12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50031501
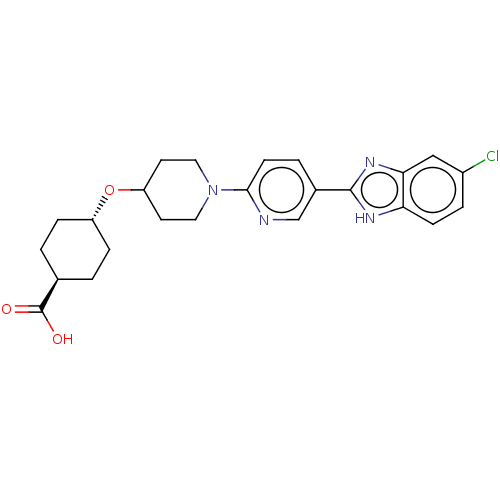
(CHEMBL3342773)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 |r,wU:6.9,wD:3.2,(37.68,-12.65,;37.68,-11.11,;39.02,-10.34,;36.35,-10.33,;36.35,-8.79,;35.02,-8.01,;33.69,-8.77,;33.68,-10.32,;35.01,-11.09,;32.35,-8,;31.02,-8.77,;31.02,-10.31,;29.69,-11.08,;28.36,-10.31,;28.35,-8.78,;29.68,-8,;27.02,-11.08,;25.69,-10.32,;24.36,-11.09,;24.36,-12.64,;25.69,-13.41,;27.03,-12.63,;23.02,-13.4,;21.62,-12.77,;20.59,-13.92,;19.05,-13.92,;18.29,-15.25,;16.75,-15.25,;19.06,-16.58,;20.59,-16.57,;21.35,-15.25,;22.86,-14.93,)| Show InChI InChI=1S/C24H27ClN4O3/c25-17-4-7-20-21(13-17)28-23(27-20)16-3-8-22(26-14-16)29-11-9-19(10-12-29)32-18-5-1-15(2-6-18)24(30)31/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,27,28)(H,30,31)/t15-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human A2A receptor |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031501

(CHEMBL3342773)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 |r,wU:6.9,wD:3.2,(37.68,-12.65,;37.68,-11.11,;39.02,-10.34,;36.35,-10.33,;36.35,-8.79,;35.02,-8.01,;33.69,-8.77,;33.68,-10.32,;35.01,-11.09,;32.35,-8,;31.02,-8.77,;31.02,-10.31,;29.69,-11.08,;28.36,-10.31,;28.35,-8.78,;29.68,-8,;27.02,-11.08,;25.69,-10.32,;24.36,-11.09,;24.36,-12.64,;25.69,-13.41,;27.03,-12.63,;23.02,-13.4,;21.62,-12.77,;20.59,-13.92,;19.05,-13.92,;18.29,-15.25,;16.75,-15.25,;19.06,-16.58,;20.59,-16.57,;21.35,-15.25,;22.86,-14.93,)| Show InChI InChI=1S/C24H27ClN4O3/c25-17-4-7-20-21(13-17)28-23(27-20)16-3-8-22(26-14-16)29-11-9-19(10-12-29)32-18-5-1-15(2-6-18)24(30)31/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,27,28)(H,30,31)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK499 from human ERG |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50031503

(CHEMBL3342775)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(ccc2[nH]1)C(F)(F)F |r,wU:6.9,wD:3.2,(29.05,-9.85,;29.05,-8.31,;30.39,-7.55,;27.72,-7.54,;27.72,-6,;26.39,-5.22,;25.06,-5.98,;25.06,-7.53,;26.38,-8.3,;23.73,-5.21,;22.39,-5.98,;22.39,-7.52,;21.07,-8.29,;19.73,-7.52,;19.72,-5.98,;21.06,-5.21,;18.4,-8.29,;17.06,-7.53,;15.73,-8.3,;15.73,-9.84,;17.06,-10.61,;18.4,-9.84,;14.4,-10.61,;12.99,-9.98,;11.96,-11.13,;10.43,-11.13,;9.66,-12.46,;10.44,-13.79,;11.97,-13.78,;12.73,-12.46,;14.23,-12.14,;8.12,-12.46,;7.35,-11.13,;7.35,-13.79,;6.58,-12.45,)| Show InChI InChI=1S/C25H27F3N4O3/c26-25(27,28)17-4-7-20-21(13-17)31-23(30-20)16-3-8-22(29-14-16)32-11-9-19(10-12-32)35-18-5-1-15(2-6-18)24(33)34/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,30,31)(H,33,34)/t15-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human A2A receptor |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021344
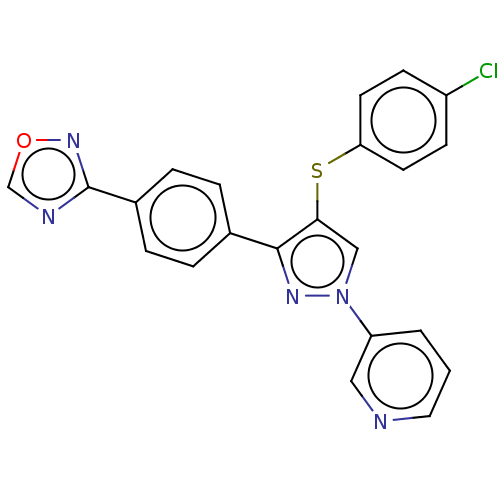
(CHEMBL3287929)Show SMILES Clc1ccc(Sc2cn(nc2-c2ccc(cc2)-c2ncon2)-c2cccnc2)cc1 Show InChI InChI=1S/C22H14ClN5OS/c23-17-7-9-19(10-8-17)30-20-13-28(18-2-1-11-24-12-18)26-21(20)15-3-5-16(6-4-15)22-25-14-29-27-22/h1-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031502
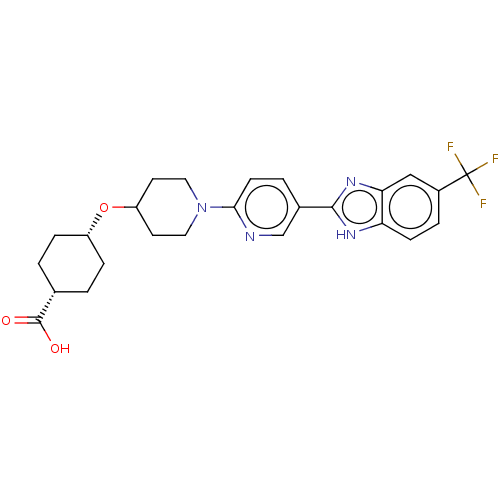
(CHEMBL3342774)Show SMILES OC(=O)[C@@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(ccc2[nH]1)C(F)(F)F |r,wU:6.9,3.2,(22.37,-7.8,;22.37,-6.26,;23.71,-5.5,;21.04,-5.48,;21.04,-3.94,;19.71,-3.16,;18.38,-3.93,;18.37,-5.48,;19.7,-6.25,;17.05,-3.16,;15.71,-3.93,;15.71,-5.47,;14.38,-6.24,;13.05,-5.47,;13.04,-3.93,;14.38,-3.16,;11.71,-6.24,;10.38,-5.47,;9.05,-6.25,;9.05,-7.79,;10.38,-8.56,;11.72,-7.79,;7.71,-8.55,;6.31,-7.93,;5.28,-9.07,;3.75,-9.07,;2.98,-10.4,;3.76,-11.73,;5.28,-11.73,;6.05,-10.4,;7.55,-10.08,;1.44,-10.4,;.67,-9.07,;.67,-11.74,;-.1,-10.4,)| Show InChI InChI=1S/C25H27F3N4O3/c26-25(27,28)17-4-7-20-21(13-17)31-23(30-20)16-3-8-22(29-14-16)32-11-9-19(10-12-32)35-18-5-1-15(2-6-18)24(33)34/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,30,31)(H,33,34)/t15-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK499 from human ERG |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50031502

(CHEMBL3342774)Show SMILES OC(=O)[C@@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(ccc2[nH]1)C(F)(F)F |r,wU:6.9,3.2,(22.37,-7.8,;22.37,-6.26,;23.71,-5.5,;21.04,-5.48,;21.04,-3.94,;19.71,-3.16,;18.38,-3.93,;18.37,-5.48,;19.7,-6.25,;17.05,-3.16,;15.71,-3.93,;15.71,-5.47,;14.38,-6.24,;13.05,-5.47,;13.04,-3.93,;14.38,-3.16,;11.71,-6.24,;10.38,-5.47,;9.05,-6.25,;9.05,-7.79,;10.38,-8.56,;11.72,-7.79,;7.71,-8.55,;6.31,-7.93,;5.28,-9.07,;3.75,-9.07,;2.98,-10.4,;3.76,-11.73,;5.28,-11.73,;6.05,-10.4,;7.55,-10.08,;1.44,-10.4,;.67,-9.07,;.67,-11.74,;-.1,-10.4,)| Show InChI InChI=1S/C25H27F3N4O3/c26-25(27,28)17-4-7-20-21(13-17)31-23(30-20)16-3-8-22(29-14-16)32-11-9-19(10-12-32)35-18-5-1-15(2-6-18)24(33)34/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,30,31)(H,33,34)/t15-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human A2A receptor |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50031505
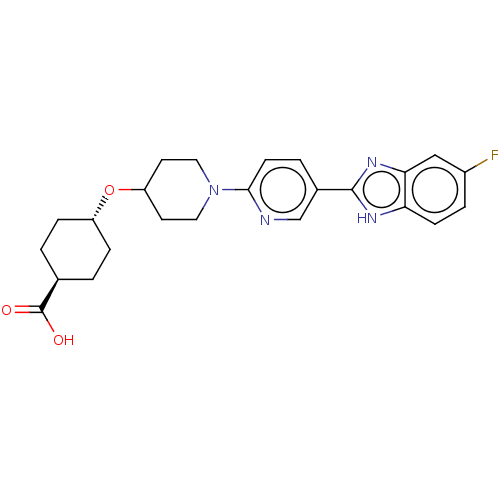
(CHEMBL3342771)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(F)ccc2[nH]1 |r,wU:6.9,wD:3.2,(37.68,-12.65,;37.68,-11.11,;39.02,-10.34,;36.35,-10.33,;36.35,-8.79,;35.02,-8.01,;33.69,-8.77,;33.68,-10.32,;35.01,-11.09,;32.35,-8,;31.02,-8.77,;31.02,-10.31,;29.69,-11.08,;28.36,-10.31,;28.35,-8.78,;29.68,-8,;27.02,-11.08,;25.69,-10.32,;24.36,-11.09,;24.36,-12.64,;25.69,-13.41,;27.03,-12.63,;23.02,-13.4,;21.62,-12.77,;20.59,-13.92,;19.05,-13.92,;18.29,-15.25,;16.75,-15.25,;19.06,-16.58,;20.59,-16.57,;21.35,-15.25,;22.86,-14.93,)| Show InChI InChI=1S/C24H27FN4O3/c25-17-4-7-20-21(13-17)28-23(27-20)16-3-8-22(26-14-16)29-11-9-19(10-12-29)32-18-5-1-15(2-6-18)24(30)31/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,27,28)(H,30,31)/t15-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human A2A receptor |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50031504

(CHEMBL3342770)Show SMILES OC(=O)[C@@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(F)ccc2[nH]1 |r,wU:6.9,3.2,(22.66,-7.8,;22.66,-6.26,;24,-5.5,;21.33,-5.48,;21.33,-3.94,;20,-3.16,;18.67,-3.93,;18.66,-5.48,;19.99,-6.25,;17.33,-3.16,;16,-3.93,;16,-5.47,;14.67,-6.24,;13.34,-5.47,;13.33,-3.93,;14.66,-3.16,;12,-6.24,;10.67,-5.47,;9.34,-6.25,;9.34,-7.79,;10.67,-8.56,;12.01,-7.79,;8,-8.55,;6.6,-7.93,;5.57,-9.07,;4.04,-9.07,;3.27,-10.4,;1.73,-10.4,;4.04,-11.73,;5.57,-11.73,;6.33,-10.4,;7.84,-10.08,)| Show InChI InChI=1S/C24H27FN4O3/c25-17-4-7-20-21(13-17)28-23(27-20)16-3-8-22(26-14-16)29-11-9-19(10-12-29)32-18-5-1-15(2-6-18)24(30)31/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,27,28)(H,30,31)/t15-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human A2A receptor |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031503

(CHEMBL3342775)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(ccc2[nH]1)C(F)(F)F |r,wU:6.9,wD:3.2,(29.05,-9.85,;29.05,-8.31,;30.39,-7.55,;27.72,-7.54,;27.72,-6,;26.39,-5.22,;25.06,-5.98,;25.06,-7.53,;26.38,-8.3,;23.73,-5.21,;22.39,-5.98,;22.39,-7.52,;21.07,-8.29,;19.73,-7.52,;19.72,-5.98,;21.06,-5.21,;18.4,-8.29,;17.06,-7.53,;15.73,-8.3,;15.73,-9.84,;17.06,-10.61,;18.4,-9.84,;14.4,-10.61,;12.99,-9.98,;11.96,-11.13,;10.43,-11.13,;9.66,-12.46,;10.44,-13.79,;11.97,-13.78,;12.73,-12.46,;14.23,-12.14,;8.12,-12.46,;7.35,-11.13,;7.35,-13.79,;6.58,-12.45,)| Show InChI InChI=1S/C25H27F3N4O3/c26-25(27,28)17-4-7-20-21(13-17)31-23(30-20)16-3-8-22(29-14-16)32-11-9-19(10-12-32)35-18-5-1-15(2-6-18)24(33)34/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,30,31)(H,33,34)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK499 from human ERG |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031504

(CHEMBL3342770)Show SMILES OC(=O)[C@@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(F)ccc2[nH]1 |r,wU:6.9,3.2,(22.66,-7.8,;22.66,-6.26,;24,-5.5,;21.33,-5.48,;21.33,-3.94,;20,-3.16,;18.67,-3.93,;18.66,-5.48,;19.99,-6.25,;17.33,-3.16,;16,-3.93,;16,-5.47,;14.67,-6.24,;13.34,-5.47,;13.33,-3.93,;14.66,-3.16,;12,-6.24,;10.67,-5.47,;9.34,-6.25,;9.34,-7.79,;10.67,-8.56,;12.01,-7.79,;8,-8.55,;6.6,-7.93,;5.57,-9.07,;4.04,-9.07,;3.27,-10.4,;1.73,-10.4,;4.04,-11.73,;5.57,-11.73,;6.33,-10.4,;7.84,-10.08,)| Show InChI InChI=1S/C24H27FN4O3/c25-17-4-7-20-21(13-17)28-23(27-20)16-3-8-22(26-14-16)29-11-9-19(10-12-29)32-18-5-1-15(2-6-18)24(30)31/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,27,28)(H,30,31)/t15-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK499 from human ERG |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031506
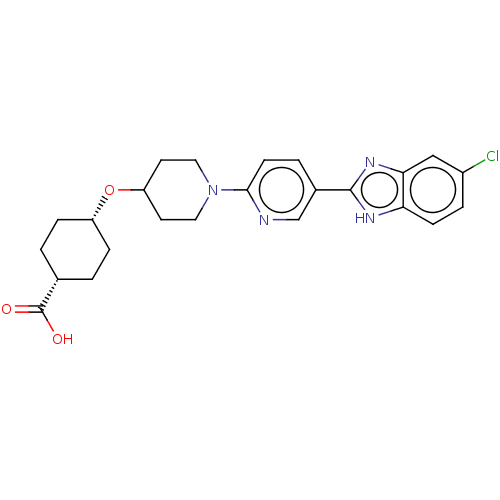
(CHEMBL3342772)Show SMILES OC(=O)[C@@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(Cl)ccc2[nH]1 |r,wU:6.9,3.2,(22.66,-7.8,;22.66,-6.26,;24,-5.5,;21.33,-5.48,;21.33,-3.94,;20,-3.16,;18.67,-3.93,;18.66,-5.48,;19.99,-6.25,;17.33,-3.16,;16,-3.93,;16,-5.47,;14.67,-6.24,;13.34,-5.47,;13.33,-3.93,;14.66,-3.16,;12,-6.24,;10.67,-5.47,;9.34,-6.25,;9.34,-7.79,;10.67,-8.56,;12.01,-7.79,;8,-8.55,;6.6,-7.93,;5.57,-9.07,;4.04,-9.07,;3.27,-10.4,;1.73,-10.4,;4.04,-11.73,;5.57,-11.73,;6.33,-10.4,;7.84,-10.08,)| Show InChI InChI=1S/C24H27ClN4O3/c25-17-4-7-20-21(13-17)28-23(27-20)16-3-8-22(26-14-16)29-11-9-19(10-12-29)32-18-5-1-15(2-6-18)24(30)31/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,27,28)(H,30,31)/t15-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK499 from human ERG |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021334
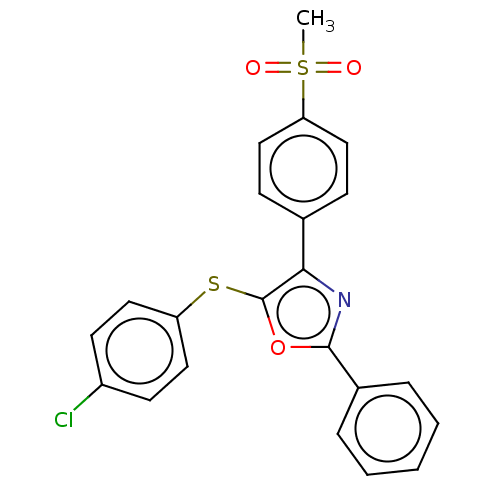
(CHEMBL3287931)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(oc1Sc1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C22H16ClNO3S2/c1-29(25,26)19-13-7-15(8-14-19)20-22(28-18-11-9-17(23)10-12-18)27-21(24-20)16-5-3-2-4-6-16/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021330
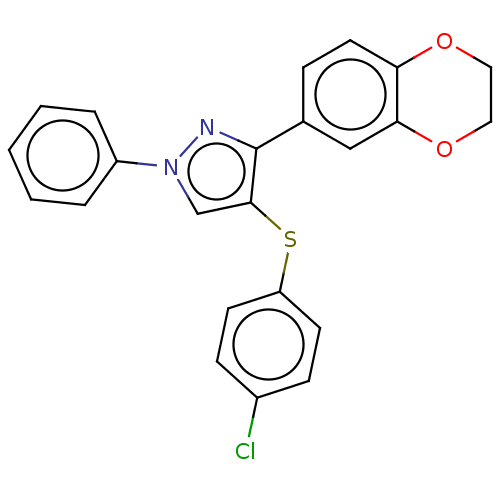
(CHEMBL3287927)Show SMILES Clc1ccc(Sc2cn(nc2-c2ccc3OCCOc3c2)-c2ccccc2)cc1 Show InChI InChI=1S/C23H17ClN2O2S/c24-17-7-9-19(10-8-17)29-22-15-26(18-4-2-1-3-5-18)25-23(22)16-6-11-20-21(14-16)28-13-12-27-20/h1-11,14-15H,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031505

(CHEMBL3342771)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)OC1CCN(CC1)c1ccc(cn1)-c1nc2cc(F)ccc2[nH]1 |r,wU:6.9,wD:3.2,(37.68,-12.65,;37.68,-11.11,;39.02,-10.34,;36.35,-10.33,;36.35,-8.79,;35.02,-8.01,;33.69,-8.77,;33.68,-10.32,;35.01,-11.09,;32.35,-8,;31.02,-8.77,;31.02,-10.31,;29.69,-11.08,;28.36,-10.31,;28.35,-8.78,;29.68,-8,;27.02,-11.08,;25.69,-10.32,;24.36,-11.09,;24.36,-12.64,;25.69,-13.41,;27.03,-12.63,;23.02,-13.4,;21.62,-12.77,;20.59,-13.92,;19.05,-13.92,;18.29,-15.25,;16.75,-15.25,;19.06,-16.58,;20.59,-16.57,;21.35,-15.25,;22.86,-14.93,)| Show InChI InChI=1S/C24H27FN4O3/c25-17-4-7-20-21(13-17)28-23(27-20)16-3-8-22(26-14-16)29-11-9-19(10-12-29)32-18-5-1-15(2-6-18)24(30)31/h3-4,7-8,13-15,18-19H,1-2,5-6,9-12H2,(H,27,28)(H,30,31)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK499 from human ERG |
ACS Med Chem Lett 5: 1082-7 (2014)
Article DOI: 10.1021/ml5003426
BindingDB Entry DOI: 10.7270/Q2WQ05CX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50314371

((1S,2R)-2-((R)-1-(3,5-bis(trifluoromethyl)phenyl)e...)Show SMILES C[C@@H](O[C@H]1CN2C(CC=CC2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,c:8| Show InChI InChI=1S/C24H20F7NO2/c1-13(15-9-16(23(26,27)28)11-17(10-15)24(29,30)31)34-20-12-32-19(3-2-4-21(32)33)22(20)14-5-7-18(25)8-6-14/h2,4-11,13,19-20,22H,3,12H2,1H3/t13-,19?,20+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 2354-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.120
BindingDB Entry DOI: 10.7270/Q2JD4XR8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220124
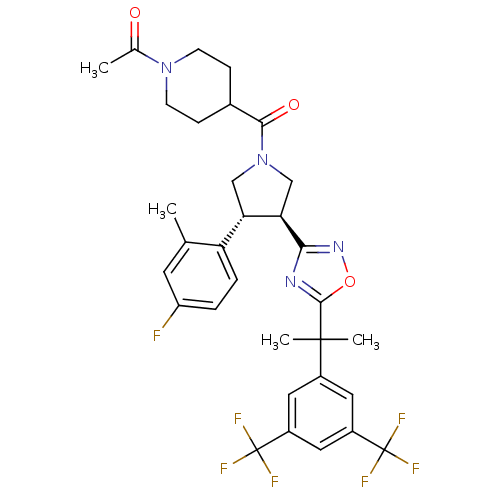
(1-(4-((3S,4R)-3-(5-(2-(3,5-bis(trifluoromethyl)phe...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1C[C@H]([C@@H](C1)c1ccc(F)cc1C)c1noc(n1)C(C)(C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C32H33F7N4O3/c1-17-11-23(33)5-6-24(17)25-15-43(28(45)19-7-9-42(10-8-19)18(2)44)16-26(25)27-40-29(46-41-27)30(3,4)20-12-21(31(34,35)36)14-22(13-20)32(37,38)39/h5-6,11-14,19,25-26H,7-10,15-16H2,1-4H3/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 5310-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.028
BindingDB Entry DOI: 10.7270/Q2DR2V75 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50314389
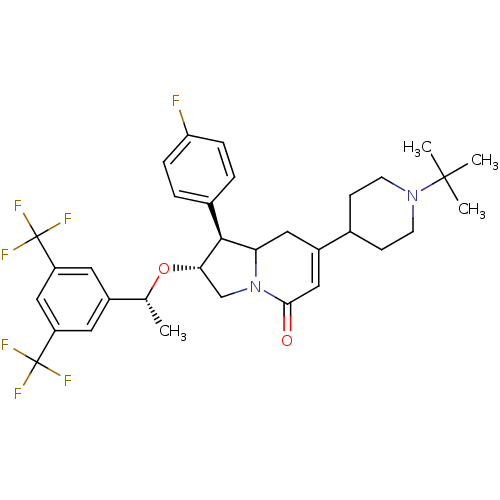
((1S,2R)-2-((R)-1-(3,5-bis(trifluoromethyl)phenyl)e...)Show SMILES C[C@@H](O[C@H]1CN2C(CC(=CC2=O)C2CCN(CC2)C(C)(C)C)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,c:8| Show InChI InChI=1S/C33H37F7N2O2/c1-19(22-13-24(32(35,36)37)17-25(14-22)33(38,39)40)44-28-18-42-27(30(28)21-5-7-26(34)8-6-21)15-23(16-29(42)43)20-9-11-41(12-10-20)31(2,3)4/h5-8,13-14,16-17,19-20,27-28,30H,9-12,15,18H2,1-4H3/t19-,27?,28+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 2354-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.120
BindingDB Entry DOI: 10.7270/Q2JD4XR8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220132

((3R,4S)-N-(3,5-bis(trifluoromethyl)benzyl)-1-(1-ac...)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1C)C(=O)C1CCN(CC1)C(C)=O Show InChI InChI=1S/C30H32F7N3O3/c1-17-10-23(31)4-5-24(17)25-15-40(27(42)20-6-8-39(9-7-20)18(2)41)16-26(25)28(43)38(3)14-19-11-21(29(32,33)34)13-22(12-19)30(35,36)37/h4-5,10-13,20,25-26H,6-9,14-16H2,1-3H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 5310-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.028
BindingDB Entry DOI: 10.7270/Q2DR2V75 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220121
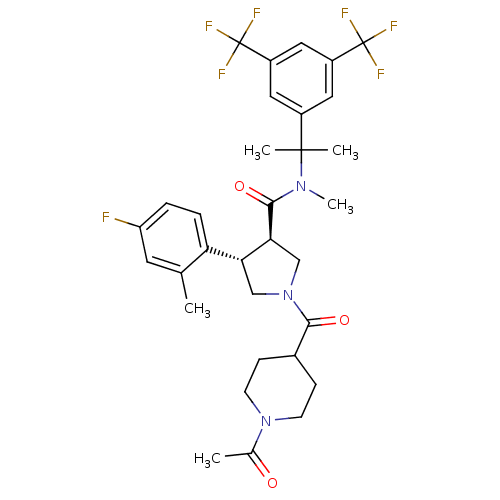
((3R,4S)-N-(2-(3,5-bis(trifluoromethyl)phenyl)propa...)Show SMILES CN(C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1C)C(=O)C1CCN(CC1)C(C)=O)C(C)(C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C32H36F7N3O3/c1-18-12-24(33)6-7-25(18)26-16-42(28(44)20-8-10-41(11-9-20)19(2)43)17-27(26)29(45)40(5)30(3,4)21-13-22(31(34,35)36)15-23(14-21)32(37,38)39/h6-7,12-15,20,26-27H,8-11,16-17H2,1-5H3/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 5310-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.028
BindingDB Entry DOI: 10.7270/Q2DR2V75 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50313630
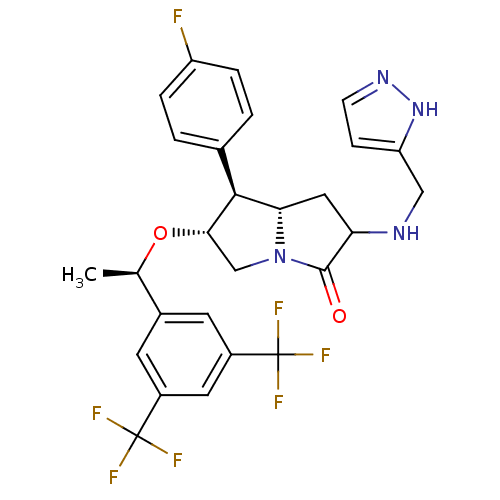
((6R,7S,7aS)-2-((1H-pyrazol-5-yl)methylamino)-6-((R...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H](CC(NCc3ccn[nH]3)C2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C27H25F7N4O2/c1-14(16-8-17(26(29,30)31)10-18(9-16)27(32,33)34)40-23-13-38-22(24(23)15-2-4-19(28)5-3-15)11-21(25(38)39)35-12-20-6-7-36-37-20/h2-10,14,21-24,35H,11-13H2,1H3,(H,36,37)/t14-,21?,22+,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 2007-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.065
BindingDB Entry DOI: 10.7270/Q23X86SP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50234162
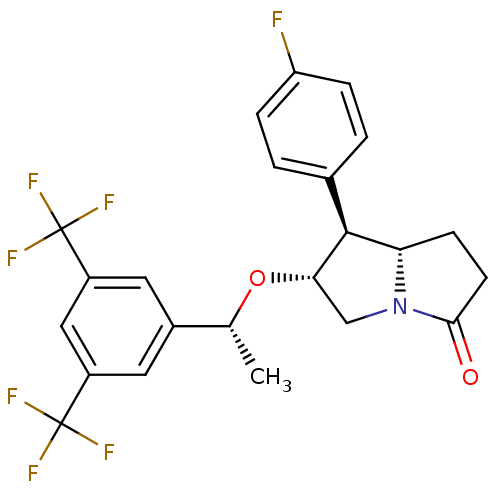
((6R,7S,7aS)-6-((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H](CCC2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H20F7NO2/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)33-19-11-31-18(6-7-20(31)32)21(19)13-2-4-17(24)5-3-13/h2-5,8-10,12,18-19,21H,6-7,11H2,1H3/t12-,18+,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 5925-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.058
BindingDB Entry DOI: 10.7270/Q2668DFC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50313627
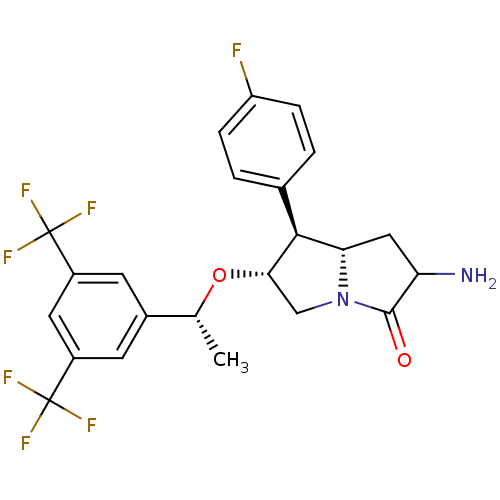
((6R,7S,7aS)-2-amino-6-((R)-1-(3,5-bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H](CC(N)C2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N2O2/c1-11(13-6-14(22(25,26)27)8-15(7-13)23(28,29)30)34-19-10-32-18(9-17(31)21(32)33)20(19)12-2-4-16(24)5-3-12/h2-8,11,17-20H,9-10,31H2,1H3/t11-,17?,18+,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 5925-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.058
BindingDB Entry DOI: 10.7270/Q2668DFC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin |
J Med Chem 52: 3039-46 (2009)
Article DOI: 10.1021/jm8016514
BindingDB Entry DOI: 10.7270/Q2FX79BT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50234162

((6R,7S,7aS)-6-((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H](CCC2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H20F7NO2/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)33-19-11-31-18(6-7-20(31)32)21(19)13-2-4-17(24)5-3-13/h2-5,8-10,12,18-19,21H,6-7,11H2,1H3/t12-,18+,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 2007-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.065
BindingDB Entry DOI: 10.7270/Q23X86SP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50218239

((3S,4R)-3-((R)-1-(3,5-bis(trifluoromethyl)phenyl)e...)Show SMILES CC(C)NC(=O)N1C[C@@H](O[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)[C@@H](C1)c1ccc(F)cc1 Show InChI InChI=1S/C24H25F7N2O2/c1-13(2)32-22(34)33-11-20(15-4-6-19(25)7-5-15)21(12-33)35-14(3)16-8-17(23(26,27)28)10-18(9-16)24(29,30)31/h4-10,13-14,20-21H,11-12H2,1-3H3,(H,32,34)/t14-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 5191-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.085
BindingDB Entry DOI: 10.7270/Q2GT5MW7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50327374
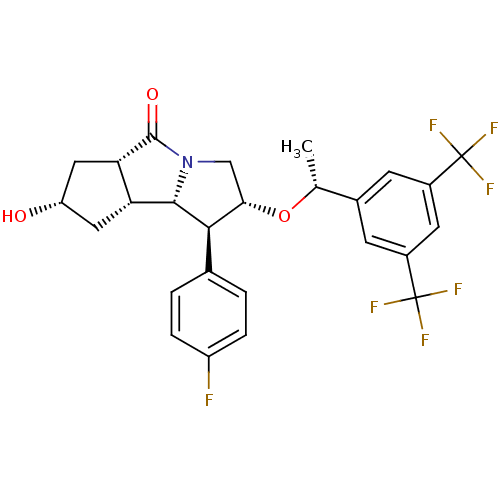
((1S,2R,5aS,7S,8aR,8bS)-2-((R)-1-(3,5-bis(trifluoro...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H]([C@@H]3C[C@H](O)C[C@@H]3C2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H24F7NO3/c1-12(14-6-15(25(28,29)30)8-16(7-14)26(31,32)33)37-21-11-34-23(19-9-18(35)10-20(19)24(34)36)22(21)13-2-4-17(27)5-3-13/h2-8,12,18-23,35H,9-11H2,1H3/t12-,18+,19-,20+,21+,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 5925-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.058
BindingDB Entry DOI: 10.7270/Q2668DFC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 56: 5940-8 (2014)
Article DOI: 10.1021/jm400751p
BindingDB Entry DOI: 10.7270/Q2ZW1N9J |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50437205

(CHEMBL2402572)Show SMILES Cc1ccccc1[C@H]1[C@@H]2CN(C[C@H]2CC[C@@H]1O[C@H](CO)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1nc(O)co1 |r| Show InChI InChI=1S/C28H28F6N2O4/c1-15-4-2-3-5-20(15)25-21-12-36(26-35-24(38)14-39-26)11-16(21)6-7-22(25)40-23(13-37)17-8-18(27(29,30)31)10-19(9-17)28(32,33)34/h2-5,8-10,14,16,21-23,25,37-38H,6-7,11-13H2,1H3/t16-,21-,22+,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 56: 5940-8 (2014)
Article DOI: 10.1021/jm400751p
BindingDB Entry DOI: 10.7270/Q2ZW1N9J |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50372480
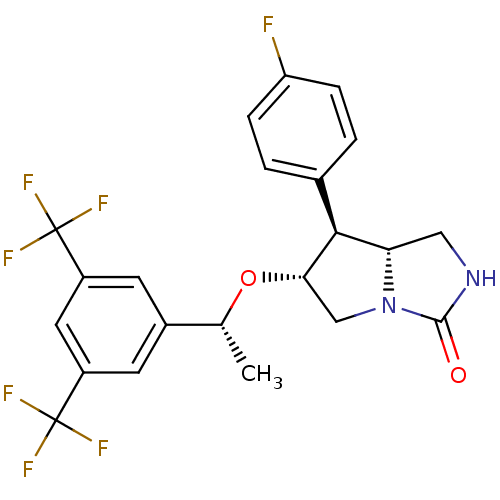
(CHEMBL270090)Show SMILES C[C@@H](O[C@H]1CN2[C@@H](CNC2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C22H19F7N2O2/c1-11(13-6-14(21(24,25)26)8-15(7-13)22(27,28)29)33-18-10-31-17(9-30-20(31)32)19(18)12-2-4-16(23)5-3-12/h2-8,11,17-19H,9-10H2,1H3,(H,30,32)/t11-,17+,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]L-703606 from human NK1 expressed in CHO cells |
Bioorg Med Chem 16: 2156-70 (2008)
Article DOI: 10.1016/j.bmc.2007.11.081
BindingDB Entry DOI: 10.7270/Q2BK1D6M |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50234162

((6R,7S,7aS)-6-((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H](CCC2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H20F7NO2/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)33-19-11-31-18(6-7-20(31)32)21(19)13-2-4-17(24)5-3-13/h2-5,8-10,12,18-19,21H,6-7,11H2,1H3/t12-,18+,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]L-703606 from human NK1 expressed in CHO cells |
Bioorg Med Chem 16: 2156-70 (2008)
Article DOI: 10.1016/j.bmc.2007.11.081
BindingDB Entry DOI: 10.7270/Q2BK1D6M |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50327384
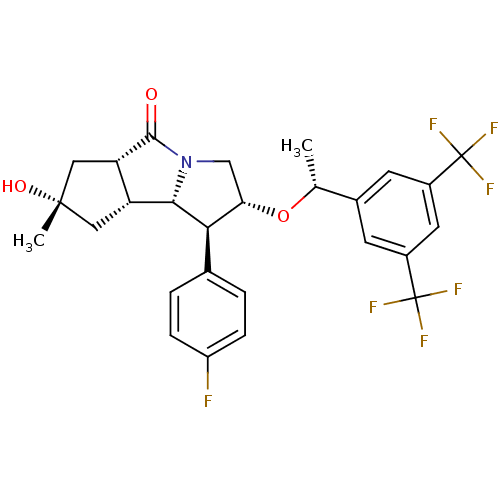
((1S,2R,5aS,7S,8aR,8bS)-2-((R)-1-(3,5-bis(trifluoro...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H]([C@@H]3C[C@](C)(O)C[C@@H]3C2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C27H26F7NO3/c1-13(15-7-16(26(29,30)31)9-17(8-15)27(32,33)34)38-21-12-35-23(22(21)14-3-5-18(28)6-4-14)19-10-25(2,37)11-20(19)24(35)36/h3-9,13,19-23,37H,10-12H2,1-2H3/t13-,19-,20+,21+,22-,23+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 5925-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.058
BindingDB Entry DOI: 10.7270/Q2668DFC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50327383
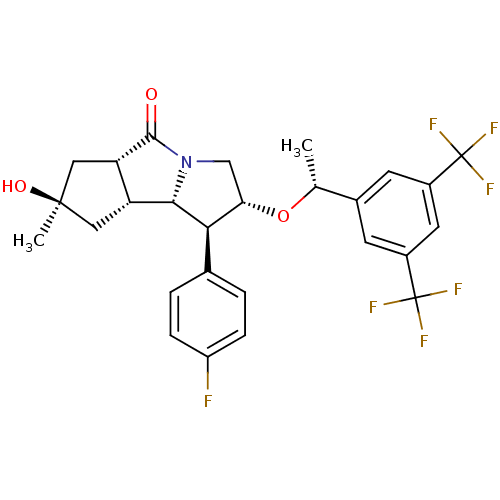
((1S,2R,5aS,7R,8aR,8bS)-2-((R)-1-(3,5-bis(trifluoro...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H]([C@@H]3C[C@@](C)(O)C[C@@H]3C2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C27H26F7NO3/c1-13(15-7-16(26(29,30)31)9-17(8-15)27(32,33)34)38-21-12-35-23(22(21)14-3-5-18(28)6-4-14)19-10-25(2,37)11-20(19)24(35)36/h3-9,13,19-23,37H,10-12H2,1-2H3/t13-,19-,20+,21+,22-,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 5925-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.058
BindingDB Entry DOI: 10.7270/Q2668DFC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50314398
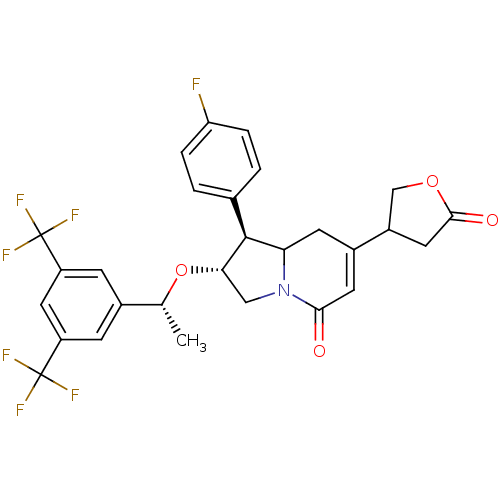
((1S,2R)-2-((R)-1-(3,5-bis(trifluoromethyl)phenyl)e...)Show SMILES C[C@@H](O[C@H]1CN2C(CC(=CC2=O)C2COC(=O)C2)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,c:8| Show InChI InChI=1S/C28H24F7NO4/c1-14(16-6-19(27(30,31)32)11-20(7-16)28(33,34)35)40-23-12-36-22(26(23)15-2-4-21(29)5-3-15)8-17(9-24(36)37)18-10-25(38)39-13-18/h2-7,9,11,14,18,22-23,26H,8,10,12-13H2,1H3/t14-,18?,22?,23+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 2354-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.120
BindingDB Entry DOI: 10.7270/Q2JD4XR8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50218225
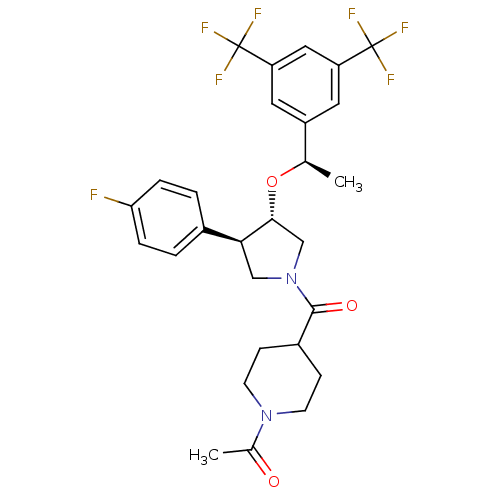
(1-(4-((3S,4R)-3-((R)-1-(3,5-bis(trifluoromethyl)ph...)Show SMILES C[C@@H](O[C@@H]1CN(C[C@H]1c1ccc(F)cc1)C(=O)C1CCN(CC1)C(C)=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H29F7N2O3/c1-16(20-11-21(27(30,31)32)13-22(12-20)28(33,34)35)40-25-15-37(14-24(25)18-3-5-23(29)6-4-18)26(39)19-7-9-36(10-8-19)17(2)38/h3-6,11-13,16,19,24-25H,7-10,14-15H2,1-2H3/t16-,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 5191-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.085
BindingDB Entry DOI: 10.7270/Q2GT5MW7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50218240
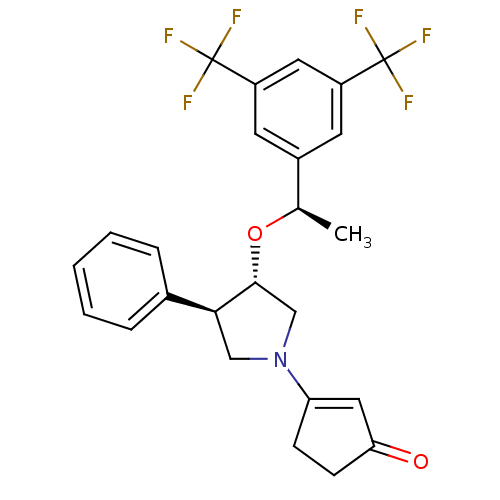
(3-((3S,4R)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...)Show SMILES C[C@@H](O[C@@H]1CN(C[C@H]1c1ccccc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |t:16| Show InChI InChI=1S/C25H23F6NO2/c1-15(17-9-18(24(26,27)28)11-19(10-17)25(29,30)31)34-23-14-32(20-7-8-21(33)12-20)13-22(23)16-5-3-2-4-6-16/h2-6,9-12,15,22-23H,7-8,13-14H2,1H3/t15-,22+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 5191-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.085
BindingDB Entry DOI: 10.7270/Q2GT5MW7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220123

(1-(4-((3R,4S)-3-(5-(2-(3,5-bis(trifluoromethyl)phe...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1C[C@@H]([C@H](C1)c1ccc(F)cc1C)c1noc(n1)C(C)(C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C32H33F7N4O3/c1-17-11-23(33)5-6-24(17)25-15-43(28(45)19-7-9-42(10-8-19)18(2)44)16-26(25)27-40-29(46-41-27)30(3,4)20-12-21(31(34,35)36)14-22(13-20)32(37,38)39/h5-6,11-14,19,25-26H,7-10,15-16H2,1-4H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 5310-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.028
BindingDB Entry DOI: 10.7270/Q2DR2V75 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220118
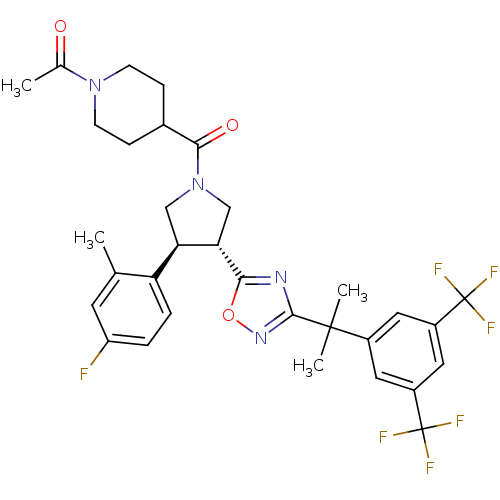
(1-(4-((3R,4S)-3-(3-(2-(3,5-bis(trifluoromethyl)phe...)Show SMILES CC(=O)N1CCC(CC1)C(=O)N1C[C@@H]([C@H](C1)c1ccc(F)cc1C)c1nc(no1)C(C)(C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C32H33F7N4O3/c1-17-11-23(33)5-6-24(17)25-15-43(28(45)19-7-9-42(10-8-19)18(2)44)16-26(25)27-40-29(41-46-27)30(3,4)20-12-21(31(34,35)36)14-22(13-20)32(37,38)39/h5-6,11-14,19,25-26H,7-10,15-16H2,1-4H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 5310-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.028
BindingDB Entry DOI: 10.7270/Q2DR2V75 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50218213
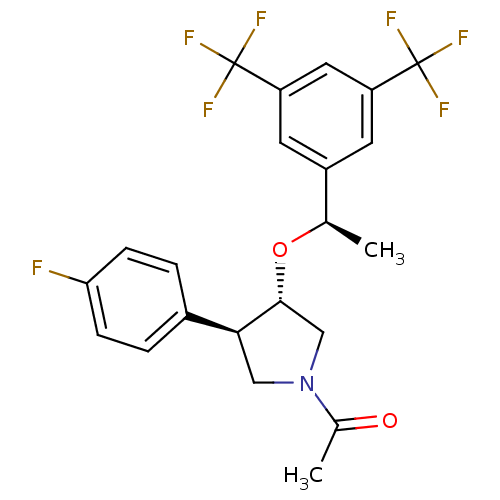
(1-((3S,4R)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...)Show SMILES C[C@@H](O[C@@H]1CN(C[C@H]1c1ccc(F)cc1)C(C)=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C22H20F7NO2/c1-12(15-7-16(21(24,25)26)9-17(8-15)22(27,28)29)32-20-11-30(13(2)31)10-19(20)14-3-5-18(23)6-4-14/h3-9,12,19-20H,10-11H2,1-2H3/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 5191-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.085
BindingDB Entry DOI: 10.7270/Q2GT5MW7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50313629
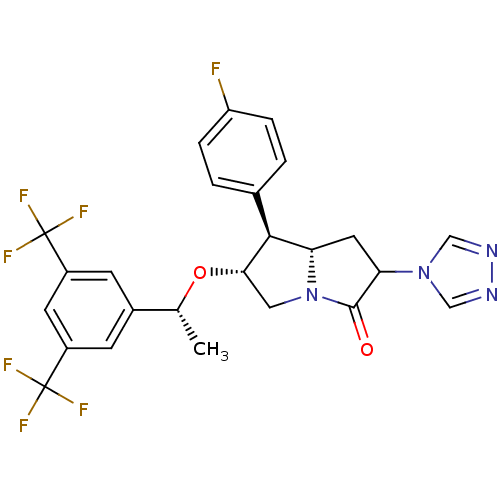
((6R,7S,7aS)-6-((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H](CC(C2=O)n2cnnc2)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H21F7N4O2/c1-13(15-6-16(24(27,28)29)8-17(7-15)25(30,31)32)38-21-10-36-19(22(21)14-2-4-18(26)5-3-14)9-20(23(36)37)35-11-33-34-12-35/h2-8,11-13,19-22H,9-10H2,1H3/t13-,19+,20?,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 2007-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.065
BindingDB Entry DOI: 10.7270/Q23X86SP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50313620

((6R,7S,7aR)-6-((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CN2[C@H]([C@@H]1c1ccc(F)cc1)C(C)(O)CC2=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H22F7NO3/c1-12(14-7-15(23(26,27)28)9-16(8-14)24(29,30)31)35-18-11-32-19(33)10-22(2,34)21(32)20(18)13-3-5-17(25)6-4-13/h3-9,12,18,20-21,34H,10-11H2,1-2H3/t12-,18+,20-,21-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 2007-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.065
BindingDB Entry DOI: 10.7270/Q23X86SP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50313620

((6R,7S,7aR)-6-((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CN2[C@H]([C@@H]1c1ccc(F)cc1)C(C)(O)CC2=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H22F7NO3/c1-12(14-7-15(23(26,27)28)9-16(8-14)24(29,30)31)35-18-11-32-19(33)10-22(2,34)21(32)20(18)13-3-5-17(25)6-4-13/h3-9,12,18,20-21,34H,10-11H2,1-2H3/t12-,18+,20-,21-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 2007-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.065
BindingDB Entry DOI: 10.7270/Q23X86SP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50313622
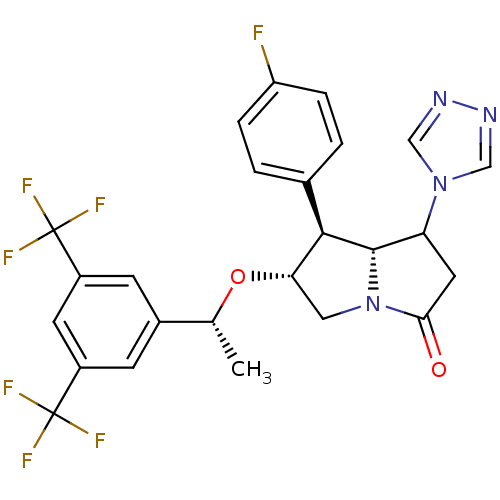
((6R,7S,7aR)-6-((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H](C(CC2=O)n2cnnc2)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H21F7N4O2/c1-13(15-6-16(24(27,28)29)8-17(7-15)25(30,31)32)38-20-10-36-21(37)9-19(35-11-33-34-12-35)23(36)22(20)14-2-4-18(26)5-3-14/h2-8,11-13,19-20,22-23H,9-10H2,1H3/t13-,19?,20+,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 2007-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.065
BindingDB Entry DOI: 10.7270/Q23X86SP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50313629

((6R,7S,7aS)-6-((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1CN2[C@@H](CC(C2=O)n2cnnc2)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H21F7N4O2/c1-13(15-6-16(24(27,28)29)8-17(7-15)25(30,31)32)38-21-10-36-19(22(21)14-2-4-18(26)5-3-14)9-20(23(36)37)35-11-33-34-12-35/h2-8,11-13,19-22H,9-10H2,1H3/t13-,19+,20?,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 2007-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.065
BindingDB Entry DOI: 10.7270/Q23X86SP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data