

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Serine/threonine-protein kinase B-raf | ||
| Ligand | BDBM50330924 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_685663 (CHEMBL1285565) | ||
| IC50 | 0.230000±n/a nM | ||
| Citation |  Wang, X; Berger, DM; Salaski, EJ; Torres, N; Dutia, M; Hanna, C; Hu, Y; Levin, JI; Powell, D; Wojciechowicz, D; Collins, K; Frommer, E; Lucas, J Indazolylpyrazolopyrimidine as highly potent B-Raf inhibitors with in vivo activity. J Med Chem53:7874-8 (2010) [PubMed] Article Wang, X; Berger, DM; Salaski, EJ; Torres, N; Dutia, M; Hanna, C; Hu, Y; Levin, JI; Powell, D; Wojciechowicz, D; Collins, K; Frommer, E; Lucas, J Indazolylpyrazolopyrimidine as highly potent B-Raf inhibitors with in vivo activity. J Med Chem53:7874-8 (2010) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Serine/threonine-protein kinase B-raf | |||
| Name: | Serine/threonine-protein kinase B-raf | ||
| Synonyms: | B-RAF | B-Raf Protein Kinase | B-Raf proto-oncogene serine/threonine-protein kinase | BRAF | BRAF1 | BRAF_HUMAN | RAFB1 | p94 | v-Raf murine sarcoma viral oncogene homolog B1 | ||
| Type: | Serine/threonine-protein kinase | ||
| Mol. Mass.: | 84446.00 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P15056 | ||
| Residue: | 766 | ||
| Sequence: |
| ||
| BDBM50330924 | |||
| n/a | |||
| Name | BDBM50330924 | ||
| Synonyms: | 7-(2-fluoro-4-((1S,4S)-5-methyl-2,5-diazabicyclo[2.2.1]heptan-2-yl)phenyl)-3-(1H-indazol-4-yl)-2-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidine | CHEMBL1276169 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C30H25FN8 | ||
| Mol. Mass. | 516.5715 | ||
| SMILES | CN1C[C@@H]2C[C@H]1CN2c1ccc(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(25.08,5.67,;25.09,4.13,;23.75,3.34,;23.77,1.8,;24.85,2.9,;26.43,3.36,;26.44,1.82,;25.1,1.04,;25.11,-.49,;26.45,-1.26,;26.45,-2.8,;25.12,-3.57,;23.78,-2.81,;22.45,-3.59,;23.77,-1.27,;25.13,-5.11,;23.8,-5.88,;23.79,-7.42,;25.13,-8.19,;26.46,-7.42,;27.94,-7.9,;28.85,-6.65,;27.94,-5.39,;26.46,-5.87,;30.39,-6.64,;31.16,-7.98,;32.7,-7.98,;33.47,-6.64,;32.69,-5.3,;31.15,-5.31,;28.41,-9.36,;29.92,-9.68,;30.4,-11.14,;29.36,-12.29,;27.85,-11.97,;26.61,-12.87,;25.37,-11.96,;25.85,-10.5,;27.39,-10.51,)| | ||
| Structure | 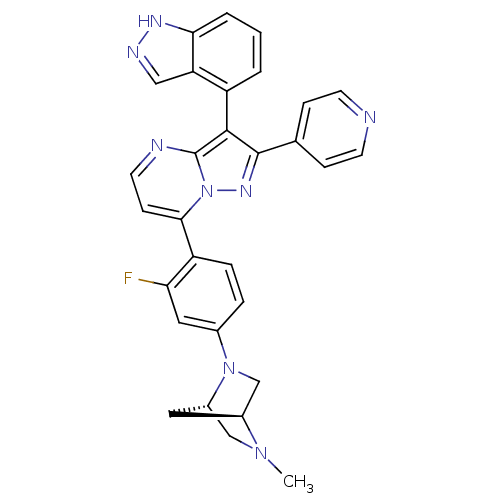
| ||