 Found 2372 hits with Last Name = 'levin' and Initial = 'ji'
Found 2372 hits with Last Name = 'levin' and Initial = 'ji' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026212
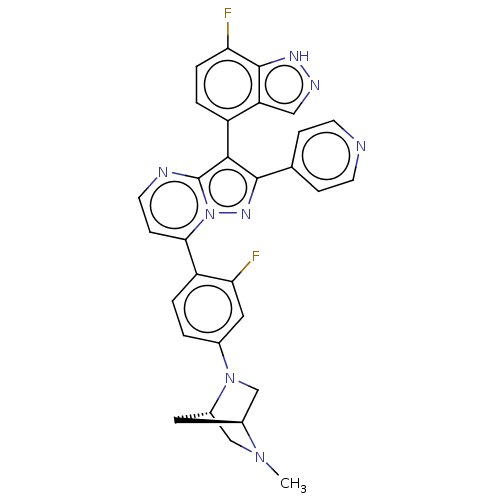
(CHEMBL1276185)Show SMILES [H][C@]12CN(c3ccc(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3ccc(F)c4[nH]ncc34)[C@]([H])(CN1C)C2 |r,wU:36.42,1.0,(5.96,-12.33,;4.87,-13.43,;4.88,-14.97,;3.54,-15.75,;3.55,-17.28,;4.89,-18.05,;4.89,-19.59,;3.56,-20.36,;2.23,-19.6,;.9,-20.38,;2.22,-18.06,;3.57,-21.9,;2.24,-22.67,;2.24,-24.21,;3.57,-24.98,;4.91,-24.21,;6.38,-24.69,;7.29,-23.44,;6.38,-22.18,;4.91,-22.66,;8.83,-23.43,;9.6,-24.77,;11.14,-24.77,;11.91,-23.43,;11.13,-22.09,;9.6,-22.1,;6.86,-26.15,;8.36,-26.47,;8.84,-27.93,;7.81,-29.09,;8.29,-30.55,;6.3,-28.76,;5.05,-29.66,;3.81,-28.75,;4.3,-27.29,;5.83,-27.3,;2.21,-14.99,;.66,-14.98,;2.2,-13.45,;3.53,-12.67,;3.53,-11.12,;3.29,-13.89,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026207
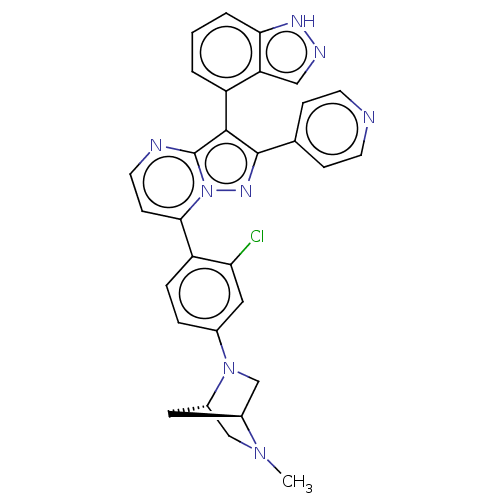
(CHEMBL1276170)Show SMILES [H][C@]12CN(c3ccc(c(Cl)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1C)C2 |r,wU:35.41,1.0,(38.98,4.96,;37.9,3.86,;37.9,2.32,;36.57,1.54,;36.58,.01,;37.92,-.76,;37.92,-2.3,;36.58,-3.07,;35.25,-2.31,;33.92,-3.09,;35.24,-.77,;36.59,-4.61,;35.26,-5.38,;35.26,-6.93,;36.6,-7.7,;37.93,-6.92,;39.41,-7.4,;40.32,-6.15,;39.41,-4.89,;37.93,-5.37,;41.86,-6.14,;42.63,-7.48,;44.17,-7.48,;44.94,-6.15,;44.16,-4.81,;42.62,-4.81,;39.88,-8.87,;41.39,-9.18,;41.87,-10.64,;40.83,-11.8,;39.32,-11.48,;38.08,-12.37,;36.84,-11.46,;37.32,-10.01,;38.86,-10.01,;35.24,2.3,;33.69,2.3,;35.22,3.84,;36.56,4.62,;36.55,6.17,;36.32,3.4,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330925
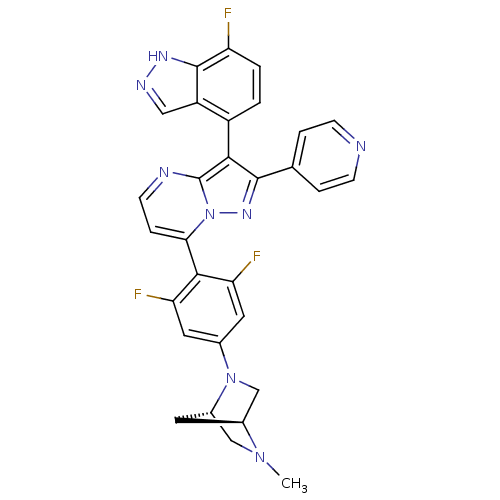
(7-(2,6-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(F)c2[nH]ncc12 |r,wU:3.3,5.4,(16.85,-11.72,;16.86,-13.27,;15.53,-14.05,;15.54,-15.59,;16.62,-14.49,;18.2,-14.03,;18.21,-15.57,;16.87,-16.35,;16.88,-17.88,;15.54,-18.66,;15.55,-20.2,;14.22,-20.98,;16.89,-20.96,;18.22,-20.19,;19.56,-20.96,;18.22,-18.65,;16.9,-22.5,;15.57,-23.27,;15.57,-24.82,;16.9,-25.59,;18.24,-24.81,;19.71,-25.29,;20.62,-24.04,;19.71,-22.78,;18.23,-23.26,;22.16,-24.03,;22.93,-25.37,;24.47,-25.37,;25.24,-24.04,;24.46,-22.7,;22.92,-22.7,;20.19,-26.76,;21.69,-27.07,;22.17,-28.53,;21.14,-29.69,;21.62,-31.15,;19.63,-29.37,;18.38,-30.26,;17.14,-29.36,;17.62,-27.9,;19.16,-27.9,)| Show InChI InChI=1S/C30H23F3N8/c1-39-14-19-10-18(39)15-40(19)17-11-23(32)27(24(33)12-17)25-6-9-35-30-26(20-2-3-22(31)29-21(20)13-36-37-29)28(38-41(25)30)16-4-7-34-8-5-16/h2-9,11-13,18-19H,10,14-15H2,1H3,(H,36,37)/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070257
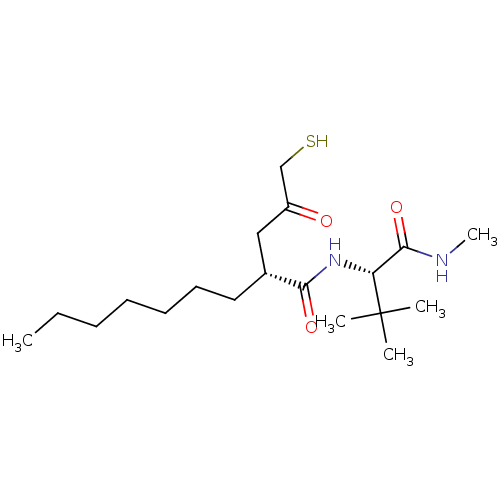
((R)-2-(3-Mercapto-2-oxo-propyl)-nonanoic acid ((S)...)Show SMILES CCCCCCC[C@H](CC(=O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C19H36N2O3S/c1-6-7-8-9-10-11-14(12-15(22)13-25)17(23)21-16(18(24)20-5)19(2,3)4/h14,16,25H,6-13H2,1-5H3,(H,20,24)(H,21,23)/t14-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-9, gelatinase-B |
Bioorg Med Chem Lett 8: 1163-8 (1999)
BindingDB Entry DOI: 10.7270/Q2348JH9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070256
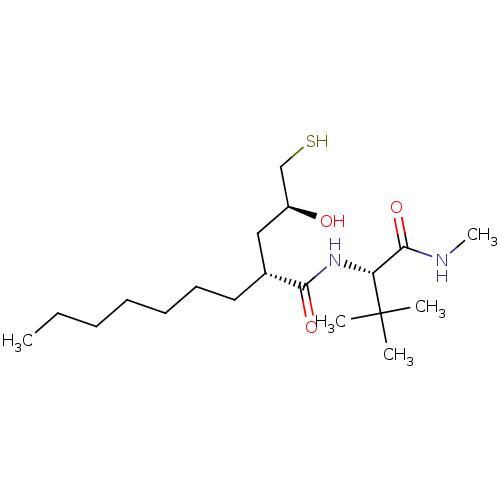
((R)-2-((S)-2-Hydroxy-3-mercapto-propyl)-nonanoic a...)Show SMILES CCCCCCC[C@H](C[C@H](O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C19H38N2O3S/c1-6-7-8-9-10-11-14(12-15(22)13-25)17(23)21-16(18(24)20-5)19(2,3)4/h14-16,22,25H,6-13H2,1-5H3,(H,20,24)(H,21,23)/t14-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-9, gelatinase-B |
Bioorg Med Chem Lett 8: 1163-8 (1999)
BindingDB Entry DOI: 10.7270/Q2348JH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330905
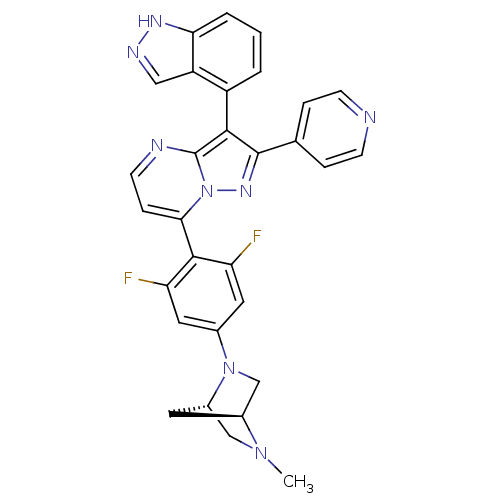
(7-(2,6-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(24.13,-33.61,;24.13,-35.15,;22.8,-35.94,;22.81,-37.47,;23.89,-36.37,;25.47,-35.92,;25.48,-37.46,;24.14,-38.23,;24.15,-39.77,;22.82,-40.55,;22.83,-42.09,;21.5,-42.86,;24.16,-42.85,;25.5,-42.07,;26.83,-42.84,;25.49,-40.53,;24.17,-44.38,;22.84,-45.15,;22.84,-46.7,;24.17,-47.47,;25.51,-46.7,;26.98,-47.18,;27.89,-45.92,;26.98,-44.67,;25.51,-45.15,;29.43,-45.92,;30.2,-47.26,;31.74,-47.26,;32.51,-45.92,;31.73,-44.58,;30.2,-44.59,;27.46,-48.64,;28.96,-48.96,;29.44,-50.42,;28.41,-51.57,;26.9,-51.25,;25.65,-52.15,;24.41,-51.24,;24.9,-49.78,;26.43,-49.79,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-20-11-19(38)16-39(20)18-12-23(31)28(24(32)13-18)26-7-10-34-30-27(21-3-2-4-25-22(21)14-35-36-25)29(37-40(26)30)17-5-8-33-9-6-17/h2-10,12-14,19-20H,11,15-16H2,1H3,(H,35,36)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330924
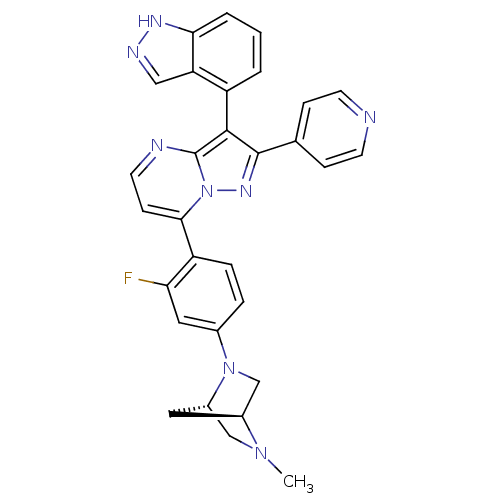
(7-(2-fluoro-4-((1S,4S)-5-methyl-2,5-diazabicyclo[2...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(25.08,5.67,;25.09,4.13,;23.75,3.34,;23.77,1.8,;24.85,2.9,;26.43,3.36,;26.44,1.82,;25.1,1.04,;25.11,-.49,;26.45,-1.26,;26.45,-2.8,;25.12,-3.57,;23.78,-2.81,;22.45,-3.59,;23.77,-1.27,;25.13,-5.11,;23.8,-5.88,;23.79,-7.42,;25.13,-8.19,;26.46,-7.42,;27.94,-7.9,;28.85,-6.65,;27.94,-5.39,;26.46,-5.87,;30.39,-6.64,;31.16,-7.98,;32.7,-7.98,;33.47,-6.64,;32.69,-5.3,;31.15,-5.31,;28.41,-9.36,;29.92,-9.68,;30.4,-11.14,;29.36,-12.29,;27.85,-11.97,;26.61,-12.87,;25.37,-11.96,;25.85,-10.5,;27.39,-10.51,)| Show InChI InChI=1S/C30H25FN8/c1-37-16-21-13-20(37)17-38(21)19-5-6-23(25(31)14-19)27-9-12-33-30-28(22-3-2-4-26-24(22)15-34-35-26)29(36-39(27)30)18-7-10-32-11-8-18/h2-12,14-15,20-21H,13,16-17H2,1H3,(H,34,35)/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11867
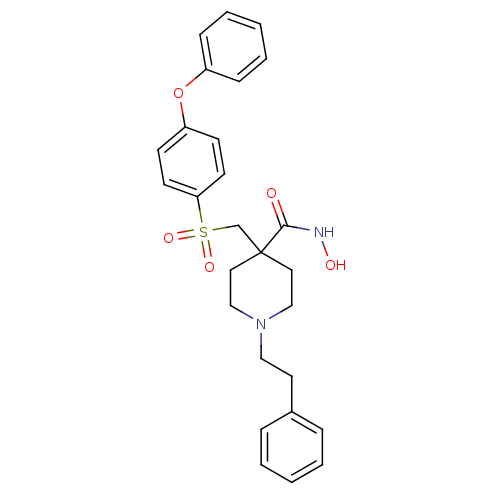
(CHEMBL234350 | N-Hydroxy-4-{[(4-phenoxyphenyl)sulf...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccccc3)cc2)CCN(CCc2ccccc2)CC1 Show InChI InChI=1S/C27H30N2O5S/c30-26(28-31)27(16-19-29(20-17-27)18-15-22-7-3-1-4-8-22)21-35(32,33)25-13-11-24(12-14-25)34-23-9-5-2-6-10-23/h1-14,31H,15-21H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 34-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.10.004
BindingDB Entry DOI: 10.7270/Q2862G2M |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070228

(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-2,2-dimet...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C18H34N2O3S/c1-6-7-8-9-10-11-13(14(21)12-24)16(22)20-15(17(23)19-5)18(2,3)4/h13,15,24H,6-12H2,1-5H3,(H,19,23)(H,20,22)/t13?,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303103
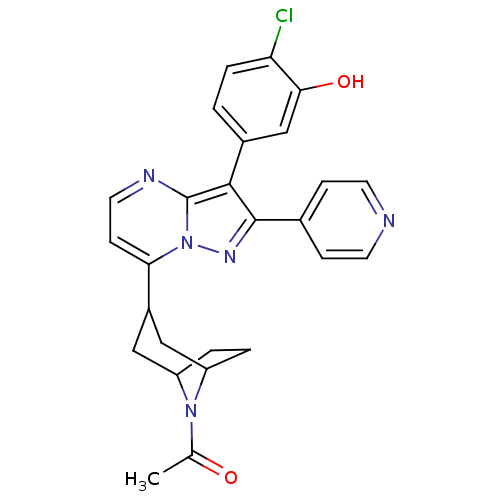
(1-(3-(3-(4-chloro-3-hydroxyphenyl)-2-(pyridin-4-yl...)Show SMILES CC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(Cl)c(O)c1 |THB:11:9:3:5.6| Show InChI InChI=1S/C26H24ClN5O2/c1-15(33)31-19-3-4-20(31)13-18(12-19)22-8-11-29-26-24(17-2-5-21(27)23(34)14-17)25(30-32(22)26)16-6-9-28-10-7-16/h2,5-11,14,18-20,34H,3-4,12-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50025947
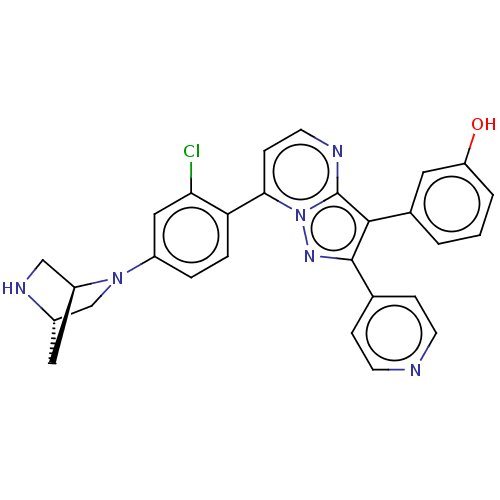
(CHEMBL1088438)Show SMILES [H][C@]12CN(c3ccc(c(Cl)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc(O)c3)[C@]([H])(CN1)C2 |r| Show InChI InChI=1S/C28H23ClN6O/c29-24-14-20(34-16-19-13-21(34)15-32-19)4-5-23(24)25-8-11-31-28-26(18-2-1-3-22(36)12-18)27(33-35(25)28)17-6-9-30-10-7-17/h1-12,14,19,21,32,36H,13,15-16H2/t19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026014
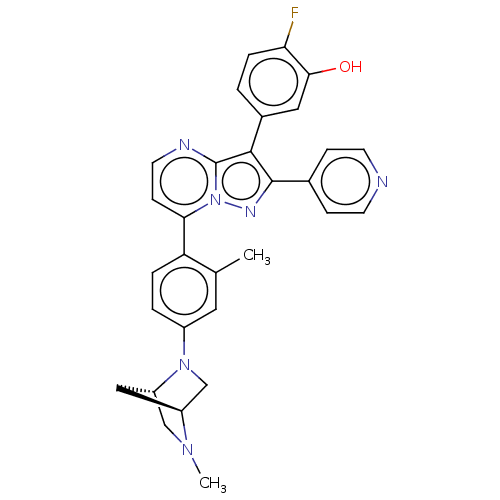
(CHEMBL1081284)Show SMILES [H][C@]12CN(c3ccc(c(C)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3ccc(F)c(O)c3)[C@]([H])(CN1C)C2 |r| Show InChI InChI=1S/C30H27FN6O/c1-18-13-21(36-17-22-15-23(36)16-35(22)2)4-5-24(18)26-9-12-33-30-28(20-3-6-25(31)27(38)14-20)29(34-37(26)30)19-7-10-32-11-8-19/h3-14,22-23,38H,15-17H2,1-2H3/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026023
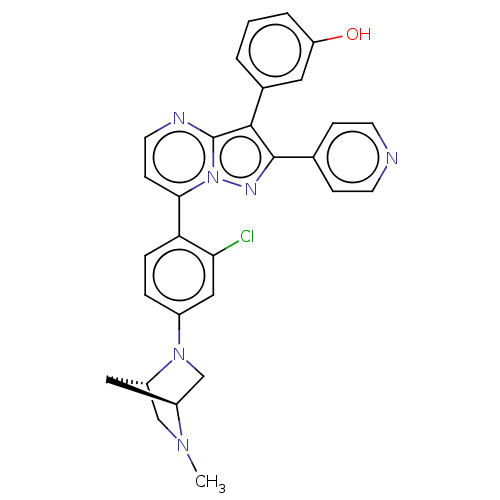
(CHEMBL1080931)Show SMILES [H][C@]12CN(c3ccc(c(Cl)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc(O)c3)[C@]([H])(CN1C)C2 |r| Show InChI InChI=1S/C29H25ClN6O/c1-34-16-22-14-21(34)17-35(22)20-5-6-24(25(30)15-20)26-9-12-32-29-27(19-3-2-4-23(37)13-19)28(33-36(26)29)18-7-10-31-11-8-18/h2-13,15,21-22,37H,14,16-17H2,1H3/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026022

(CHEMBL1081283)Show SMILES [H][C@]12CN(c3ccc(cc3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3ccc(F)c(O)c3)[C@]([H])(CN1C)C2 |r| Show InChI InChI=1S/C29H25FN6O/c1-34-16-23-15-22(34)17-35(23)21-5-2-18(3-6-21)25-10-13-32-29-27(20-4-7-24(30)26(37)14-20)28(33-36(25)29)19-8-11-31-12-9-19/h2-14,22-23,37H,15-17H2,1H3/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026210

(CHEMBL1276196)Show SMILES [H][C@]12CN(c3cc(F)c(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1C1CCC1)C2 |r,wU:36.42,1.0,(4.86,-17.8,;3.77,-18.9,;3.78,-20.44,;2.44,-21.22,;2.45,-22.75,;1.12,-23.53,;1.13,-25.07,;-.2,-25.85,;2.46,-25.83,;3.8,-25.06,;5.13,-25.82,;3.79,-23.52,;2.47,-27.37,;1.14,-28.14,;1.14,-29.68,;2.47,-30.45,;3.81,-29.68,;5.28,-30.16,;6.19,-28.9,;5.28,-27.65,;3.81,-28.13,;7.73,-28.9,;8.5,-30.24,;10.04,-30.24,;10.81,-28.9,;10.03,-27.56,;8.5,-27.57,;5.76,-31.62,;7.26,-31.94,;7.74,-33.4,;6.71,-34.55,;5.2,-34.23,;3.95,-35.13,;2.71,-34.22,;3.2,-32.76,;4.73,-32.77,;1.11,-20.46,;-.44,-20.45,;1.1,-18.92,;2.43,-18.13,;2.43,-16.59,;3.51,-15.5,;2.42,-14.42,;1.33,-15.51,;2.19,-19.36,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50025938

(CHEMBL1088439)Show SMILES [H][C@]12CN(c3ccc(cc3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3ccc(F)c(O)c3)[C@]([H])(CN1)C2 |r| Show InChI InChI=1S/C28H23FN6O/c29-23-6-3-19(13-25(23)36)26-27(18-7-10-30-11-8-18)33-35-24(9-12-31-28(26)35)17-1-4-21(5-2-17)34-16-20-14-22(34)15-32-20/h1-13,20,22,32,36H,14-16H2/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026013
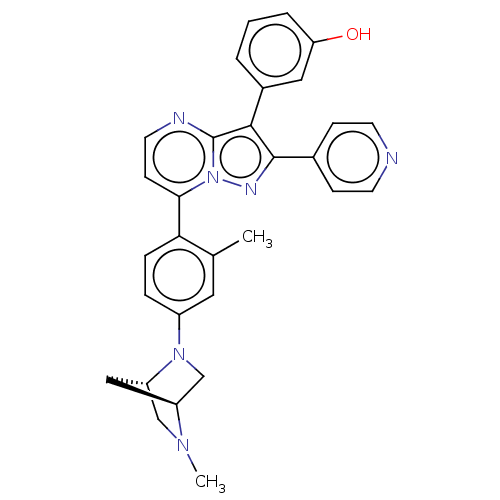
(CHEMBL1086882)Show SMILES [H][C@]12CN(c3ccc(c(C)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc(O)c3)[C@]([H])(CN1C)C2 |r| Show InChI InChI=1S/C30H28N6O/c1-19-14-22(35-18-23-16-24(35)17-34(23)2)6-7-26(19)27-10-13-32-30-28(21-4-3-5-25(37)15-21)29(33-36(27)30)20-8-11-31-12-9-20/h3-15,23-24,37H,16-18H2,1-2H3/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50025981
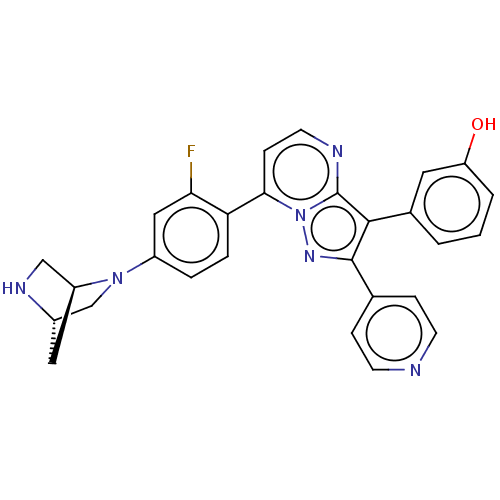
(CHEMBL1088437)Show SMILES [H][C@]12CN(c3ccc(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc(O)c3)[C@]([H])(CN1)C2 |r| Show InChI InChI=1S/C28H23FN6O/c29-24-14-20(34-16-19-13-21(34)15-32-19)4-5-23(24)25-8-11-31-28-26(18-2-1-3-22(36)12-18)27(33-35(25)28)17-6-9-30-10-7-17/h1-12,14,19,21,32,36H,13,15-16H2/t19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026010
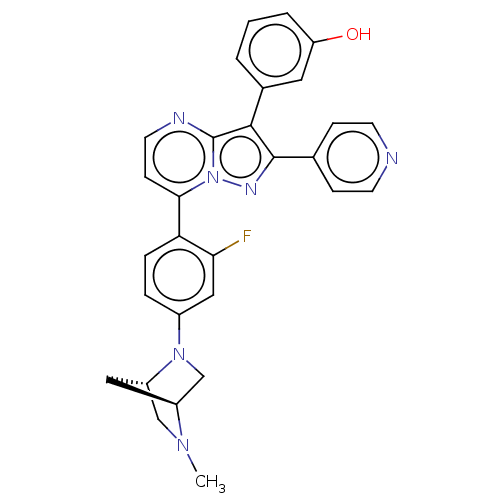
(CHEMBL1087013)Show SMILES [H][C@]12CN(c3ccc(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc(O)c3)[C@]([H])(CN1C)C2 |r| Show InChI InChI=1S/C29H25FN6O/c1-34-16-22-14-21(34)17-35(22)20-5-6-24(25(30)15-20)26-9-12-32-29-27(19-3-2-4-23(37)13-19)28(33-36(26)29)18-7-10-31-11-8-18/h2-13,15,21-22,37H,14,16-17H2,1H3/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026030
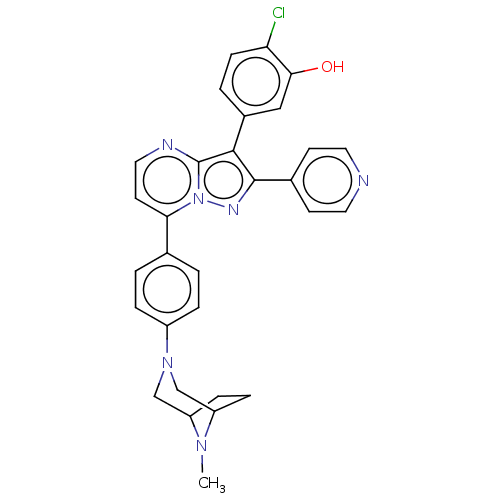
(CHEMBL1080833)Show SMILES CN1C2CCC1CN(C2)c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(Cl)c(O)c1 |TLB:0:1:3.4:8.7.6| Show InChI InChI=1S/C30H27ClN6O/c1-35-23-7-8-24(35)18-36(17-23)22-5-2-19(3-6-22)26-12-15-33-30-28(21-4-9-25(31)27(38)16-21)29(34-37(26)30)20-10-13-32-14-11-20/h2-6,9-16,23-24,38H,7-8,17-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026028
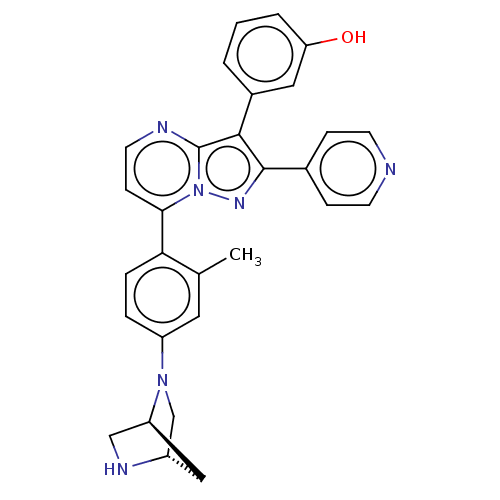
(CHEMBL1087001)Show SMILES [H][C@]12CN(c3ccc(c(C)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc(O)c3)[C@]([H])(CN1)C2 |r| Show InChI InChI=1S/C29H26N6O/c1-18-13-22(34-17-21-15-23(34)16-32-21)5-6-25(18)26-9-12-31-29-27(20-3-2-4-24(36)14-20)28(33-35(26)29)19-7-10-30-11-8-19/h2-14,21,23,32,36H,15-17H2,1H3/t21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026208
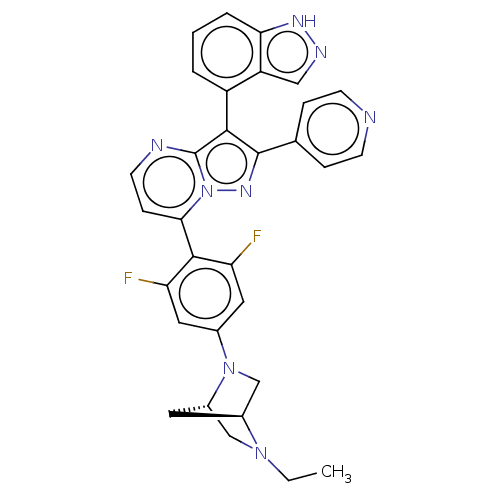
(CHEMBL1276193)Show SMILES [H][C@]12CN(c3cc(F)c(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1CC)C2 |r,wU:36.42,1.0,(-2.84,3.86,;-3.92,2.75,;-3.91,1.21,;-5.25,.44,;-5.24,-1.09,;-6.58,-1.88,;-6.57,-3.41,;-7.9,-4.19,;-5.23,-4.18,;-3.9,-3.4,;-2.56,-4.17,;-3.9,-1.86,;-5.22,-5.71,;-6.55,-6.48,;-6.55,-8.03,;-5.22,-8.8,;-3.89,-8.03,;-2.41,-8.51,;-1.5,-7.25,;-2.41,-6,;-3.89,-6.48,;.04,-7.25,;.81,-8.59,;2.35,-8.59,;3.12,-7.25,;2.34,-5.91,;.8,-5.92,;-1.93,-9.97,;-.43,-10.29,;.05,-11.75,;-.98,-12.9,;-2.49,-12.58,;-3.74,-13.48,;-4.98,-12.57,;-4.5,-11.11,;-2.96,-11.12,;-6.58,1.2,;-8.13,1.2,;-6.59,2.74,;-5.26,3.52,;-5.27,5.06,;-3.94,5.84,;-5.5,2.3,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026209

(CHEMBL1276171)Show SMILES [H][C@]12CN(c3ccc(c(Br)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1C)C2 |r,wU:35.41,1.0,(-8.28,-15.09,;-9.36,-16.19,;-9.36,-17.73,;-10.69,-18.5,;-10.68,-20.03,;-9.35,-20.8,;-9.34,-22.34,;-10.68,-23.12,;-12.01,-22.36,;-13.34,-23.13,;-12.03,-20.82,;-10.67,-24.65,;-12.01,-25.42,;-12.01,-26.97,;-10.67,-27.74,;-9.33,-26.97,;-7.86,-27.45,;-6.94,-26.19,;-7.86,-24.94,;-9.33,-25.42,;-5.41,-26.19,;-4.64,-27.53,;-3.1,-27.53,;-2.33,-26.19,;-3.1,-24.85,;-4.64,-24.86,;-7.38,-28.91,;-5.88,-29.23,;-5.4,-30.69,;-6.43,-31.84,;-7.94,-31.52,;-9.19,-32.42,;-10.42,-31.51,;-9.94,-30.05,;-8.41,-30.06,;-12.04,-17.74,;-13.58,-17.74,;-12.05,-16.21,;-10.7,-15.42,;-10.71,-13.88,;-10.94,-16.64,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026205
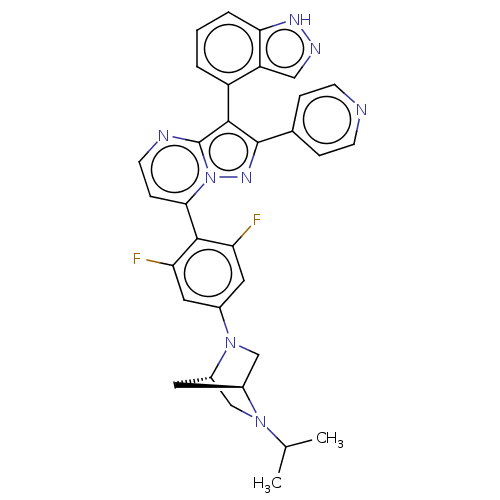
(CHEMBL1276194)Show SMILES [H][C@]12CN(c3cc(F)c(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1C(C)C)C2 |r,wU:36.42,1.0,(9.92,3.92,;8.84,2.82,;8.84,1.28,;7.51,.5,;7.52,-1.03,;6.18,-1.82,;6.19,-3.35,;4.86,-4.13,;7.52,-4.11,;8.86,-3.34,;10.2,-4.11,;8.85,-1.8,;7.53,-5.65,;6.2,-6.42,;6.2,-7.97,;7.54,-8.74,;8.87,-7.97,;10.35,-8.44,;11.26,-7.19,;10.34,-5.93,;8.87,-6.41,;12.79,-7.19,;13.56,-8.52,;15.1,-8.52,;15.88,-7.19,;15.1,-5.85,;13.56,-5.85,;10.82,-9.91,;12.32,-10.22,;12.8,-11.68,;11.77,-12.84,;10.26,-12.52,;9.02,-13.41,;7.78,-12.51,;8.26,-11.05,;9.79,-11.06,;6.17,1.26,;4.63,1.26,;6.16,2.8,;7.5,3.58,;7.49,5.12,;8.82,5.9,;6.15,5.89,;7.26,2.36,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026211

(CHEMBL1276184)Show SMILES [H][C@]12CN(c3ccc(c(C)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3ccc(F)c4[nH]ncc34)[C@]([H])(CN1C)C2 |r,wU:36.42,1.0,(-6.99,-8.62,;-8.07,-9.72,;-8.07,-11.26,;-9.4,-12.03,;-9.39,-13.57,;-8.05,-14.33,;-8.05,-15.87,;-9.39,-16.65,;-10.72,-15.89,;-12.05,-16.66,;-10.73,-14.35,;-9.38,-18.18,;-10.71,-18.95,;-10.71,-20.5,;-9.37,-21.27,;-8.04,-20.5,;-6.56,-20.98,;-5.65,-19.72,;-6.56,-18.47,;-8.04,-18.95,;-4.11,-19.72,;-3.34,-21.06,;-1.8,-21.06,;-1.03,-19.72,;-1.81,-18.38,;-3.35,-18.39,;-6.09,-22.44,;-4.59,-22.76,;-4.11,-24.22,;-5.14,-25.37,;-4.66,-26.84,;-6.65,-25.05,;-7.89,-25.95,;-9.13,-25.04,;-8.65,-23.58,;-7.11,-23.59,;-10.73,-11.27,;-12.28,-11.27,;-10.75,-9.74,;-9.41,-8.95,;-9.42,-7.41,;-9.65,-10.17,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026206
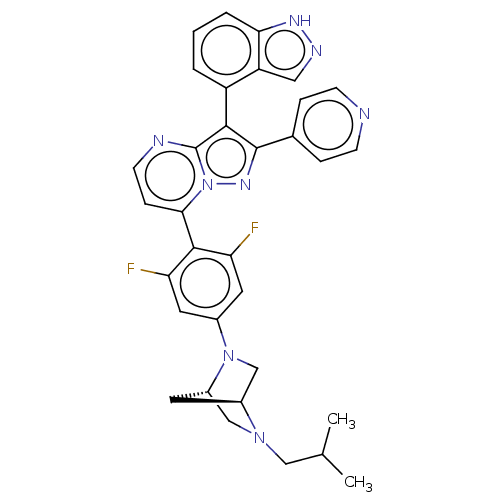
(CHEMBL1276195)Show SMILES [H][C@]12CN(c3cc(F)c(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1CC(C)C)C2 |r,wU:36.42,1.0,(21.41,.41,;20.33,-.69,;20.34,-2.23,;19,-3.01,;19.01,-4.54,;17.67,-5.32,;17.69,-6.86,;16.35,-7.64,;19.02,-7.62,;20.35,-6.85,;21.69,-7.62,;20.35,-5.31,;19.03,-9.16,;17.7,-9.93,;17.7,-11.48,;19.03,-12.25,;20.37,-11.47,;21.84,-11.95,;22.75,-10.7,;21.84,-9.44,;20.36,-9.92,;24.29,-10.69,;25.06,-12.03,;26.6,-12.03,;27.37,-10.7,;26.59,-9.36,;25.06,-9.36,;22.32,-13.42,;23.82,-13.73,;24.3,-15.19,;23.27,-16.35,;21.76,-16.03,;20.51,-16.92,;19.27,-16.01,;19.75,-14.56,;21.29,-14.56,;17.67,-2.25,;16.12,-2.25,;17.66,-.71,;18.99,.07,;18.98,1.62,;20.31,2.39,;20.31,3.93,;21.65,1.63,;18.75,-1.15,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330923
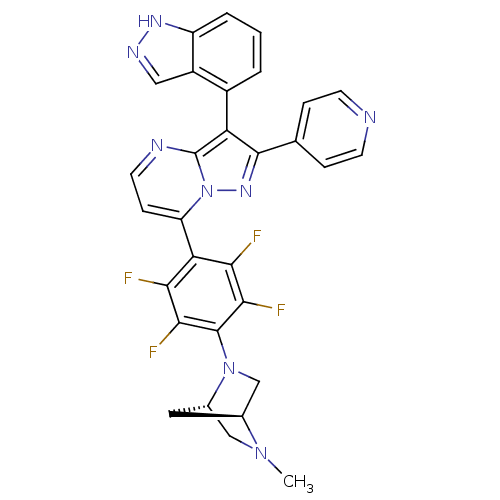
(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)-7-(2,3,5,6-te...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1c(F)c(F)c(c(F)c1F)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(-7.9,5.85,;-7.89,4.3,;-9.22,3.52,;-9.21,1.98,;-8.13,3.08,;-6.55,3.54,;-6.54,2,;-7.88,1.22,;-7.87,-.31,;-6.53,-1.08,;-5.2,-.3,;-6.53,-2.62,;-5.19,-3.39,;-7.86,-3.39,;-9.19,-2.63,;-10.52,-3.41,;-9.2,-1.09,;-10.54,-.33,;-7.85,-4.93,;-9.18,-5.7,;-9.18,-7.24,;-7.85,-8.02,;-6.51,-7.24,;-5.04,-7.72,;-4.13,-6.47,;-5.04,-5.21,;-6.51,-5.69,;-2.59,-6.46,;-1.82,-7.8,;-.28,-7.8,;.49,-6.46,;-.29,-5.13,;-1.82,-5.13,;-4.56,-9.19,;-3.06,-9.5,;-2.58,-10.96,;-3.61,-12.12,;-5.12,-11.8,;-6.37,-12.69,;-7.61,-11.78,;-7.13,-10.33,;-5.59,-10.33,)| Show InChI InChI=1S/C30H22F4N8/c1-40-13-17-11-16(40)14-41(17)29-26(33)24(31)23(25(32)27(29)34)21-7-10-36-30-22(18-3-2-4-20-19(18)12-37-38-20)28(39-42(21)30)15-5-8-35-9-6-15/h2-10,12,16-17H,11,13-14H2,1H3,(H,37,38)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50311985
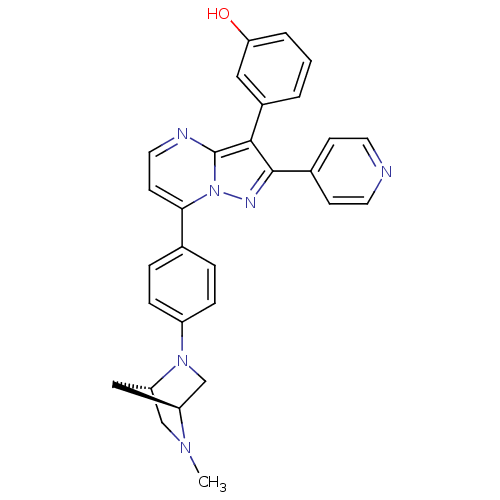
(3-(7-(4-((1S,4S)-5-methyl-2,5-diazabicyclo[2.2.1]h...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc(O)c1 |r| Show InChI InChI=1S/C29H26N6O/c1-33-17-24-16-23(33)18-34(24)22-7-5-19(6-8-22)26-11-14-31-29-27(21-3-2-4-25(36)15-21)28(32-35(26)29)20-9-12-30-13-10-20/h2-15,23-24,36H,16-18H2,1H3/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330922
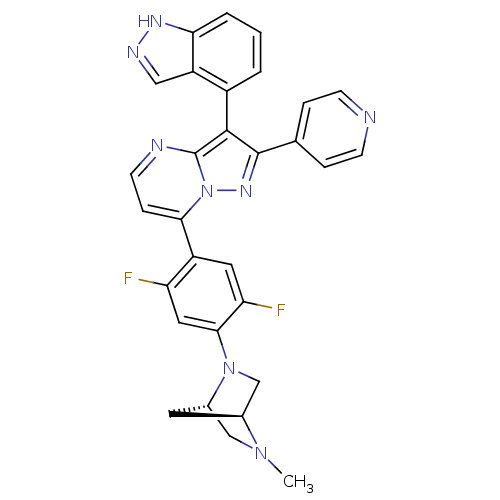
(7-(2,5-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(cc1F)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(12.11,-32.8,;12.12,-34.34,;10.78,-35.12,;10.8,-36.66,;11.88,-35.56,;13.46,-35.1,;13.47,-36.64,;12.13,-37.42,;12.14,-38.95,;10.8,-39.73,;10.81,-41.27,;9.48,-42.05,;12.15,-42.03,;13.48,-41.26,;13.48,-39.72,;14.81,-38.95,;12.16,-43.57,;10.83,-44.34,;10.82,-45.89,;12.16,-46.66,;13.49,-45.88,;14.97,-46.36,;15.88,-45.11,;14.97,-43.85,;13.49,-44.33,;17.42,-45.1,;18.19,-46.44,;19.73,-46.44,;20.5,-45.11,;19.72,-43.77,;18.18,-43.77,;15.44,-47.83,;16.95,-48.14,;17.43,-49.6,;16.39,-50.76,;14.88,-50.44,;13.64,-51.33,;12.4,-50.42,;12.88,-48.97,;14.42,-48.97,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-19-11-18(38)16-39(19)27-13-23(31)21(12-24(27)32)26-7-10-34-30-28(20-3-2-4-25-22(20)14-35-36-25)29(37-40(26)30)17-5-8-33-9-6-17/h2-10,12-14,18-19H,11,15-16H2,1H3,(H,35,36)/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50311985

(3-(7-(4-((1S,4S)-5-methyl-2,5-diazabicyclo[2.2.1]h...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc(O)c1 |r| Show InChI InChI=1S/C29H26N6O/c1-33-17-24-16-23(33)18-34(24)22-7-5-19(6-8-22)26-11-14-31-29-27(21-3-2-4-25(36)15-21)28(32-35(26)29)20-9-12-30-13-10-20/h2-15,23-24,36H,16-18H2,1H3/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173235

((S)-2-{4'-[(5-Bromo-4-methoxy-benzofuran-2-carbony...)Show SMILES COc1c(Br)ccc2oc(cc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C27H25BrN2O7S/c1-15(2)24(27(32)33)30-38(34,35)19-10-6-17(7-11-19)16-4-8-18(9-5-16)29-26(31)23-14-20-22(37-23)13-12-21(28)25(20)36-3/h4-15,24,30H,1-3H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118985
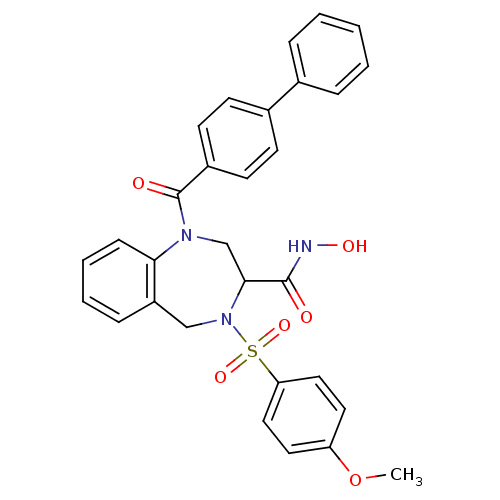
(1-(Biphenyl-4-carbonyl)-4-(4-methoxy-benzenesulfon...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C30H27N3O6S/c1-39-25-15-17-26(18-16-25)40(37,38)33-19-24-9-5-6-10-27(24)32(20-28(33)29(34)31-36)30(35)23-13-11-22(12-14-23)21-7-3-2-4-8-21/h2-18,28,36H,19-20H2,1H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118983
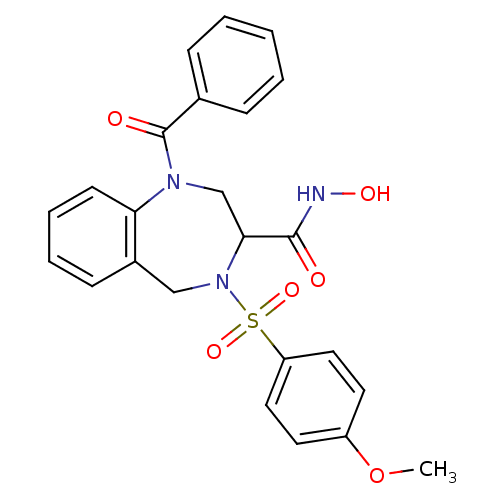
(1-Benzoyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-te...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccccc1 Show InChI InChI=1S/C24H23N3O6S/c1-33-19-11-13-20(14-12-19)34(31,32)27-15-18-9-5-6-10-21(18)26(16-22(27)23(28)25-30)24(29)17-7-3-2-4-8-17/h2-14,22,30H,15-16H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303123

(CHEMBL570681 | ethyl 3-(3-(7-fluoro-1H-indazol-4-y...)Show SMILES CCOC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(F)c2[nH]ncc12 |THB:13:11:5:7.8,(15.45,-16.9,;14.13,-17.7,;12.78,-16.95,;11.46,-17.75,;10.11,-17,;11.49,-19.29,;10.96,-20.75,;9.44,-21.43,;10.81,-21.4,;12,-20.54,;13.54,-21.38,;13,-22.85,;12.55,-21.67,;13,-24.39,;11.67,-25.16,;11.67,-26.71,;13,-27.48,;14.34,-26.71,;15.81,-27.18,;16.72,-25.93,;15.81,-24.68,;14.34,-25.16,;18.26,-25.93,;19.02,-27.26,;20.56,-27.26,;21.33,-25.93,;20.55,-24.59,;19.02,-24.6,;15.88,-28.72,;14.58,-29.54,;14.64,-31.08,;16,-31.8,;16.06,-33.34,;17.31,-30.98,;18.8,-31.39,;19.66,-30.1,;18.69,-28.88,;17.24,-29.43,)| Show InChI InChI=1S/C28H26FN7O2/c1-2-38-28(37)35-18-3-4-19(35)14-17(13-18)23-9-12-31-27-24(20-5-6-22(29)26-21(20)15-32-33-26)25(34-36(23)27)16-7-10-30-11-8-16/h5-12,15,17-19H,2-4,13-14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303121
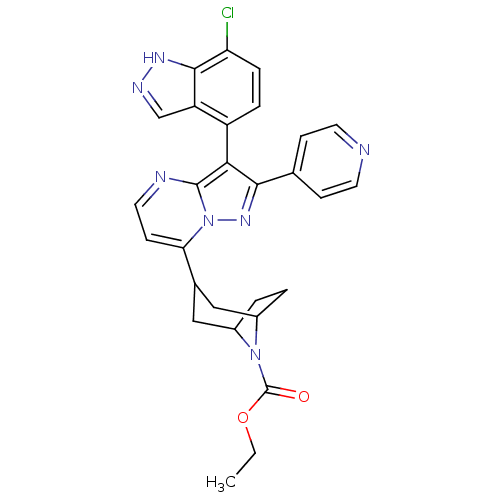
(CHEMBL585144 | ethyl 3-(3-(7-chloro-1H-indazol-4-y...)Show SMILES CCOC(=O)N1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(Cl)c2[nH]ncc12 |THB:13:11:5:7.8,(29.95,2.2,;28.63,1.41,;27.29,2.16,;25.97,1.36,;24.62,2.11,;26,-.18,;25.46,-1.65,;23.94,-2.33,;25.31,-2.29,;26.51,-1.43,;28.04,-2.27,;27.5,-3.75,;27.06,-2.56,;27.51,-5.29,;26.18,-6.06,;26.18,-7.6,;27.51,-8.37,;28.84,-7.6,;30.32,-8.07,;31.23,-6.82,;30.31,-5.57,;28.84,-6.05,;32.76,-6.82,;33.53,-8.16,;35.07,-8.16,;35.84,-6.82,;35.06,-5.48,;33.52,-5.49,;30.38,-9.61,;29.08,-10.43,;29.14,-11.97,;30.5,-12.69,;30.56,-14.23,;31.81,-11.87,;33.31,-12.28,;34.16,-10.99,;33.2,-9.78,;31.74,-10.32,)| Show InChI InChI=1S/C28H26ClN7O2/c1-2-38-28(37)35-18-3-4-19(35)14-17(13-18)23-9-12-31-27-24(20-5-6-22(29)26-21(20)15-32-33-26)25(34-36(23)27)16-7-10-30-11-8-16/h5-12,15,17-19H,2-4,13-14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303098
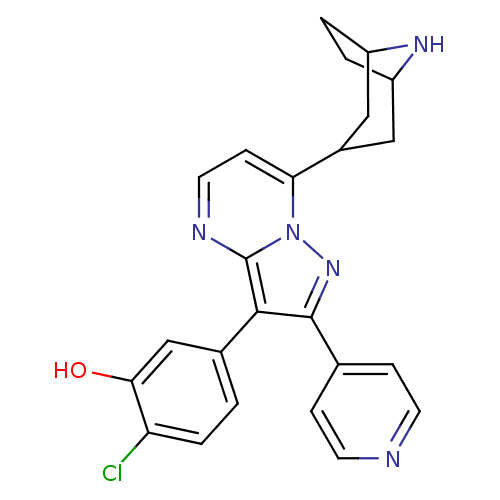
(5-(7-(8-azabicyclo[3.2.1]octan-3-yl)-2-(pyridin-4-...)Show SMILES Oc1cc(ccc1Cl)-c1c(nn2c(ccnc12)C1CC2CCC(C1)N2)-c1ccncc1 |TLB:12:17:24:20.21| Show InChI InChI=1S/C24H22ClN5O/c25-19-4-1-15(13-21(19)31)22-23(14-5-8-26-9-6-14)29-30-20(7-10-27-24(22)30)16-11-17-2-3-18(12-16)28-17/h1,4-10,13,16-18,28,31H,2-3,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330921

((1S,4S)-2-(4-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)...)Show SMILES CN1C[C@@H]2CC[C@H]1CN2c1ccc(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wD:6.6,3.9,(4.83,5.26,;4.84,3.71,;3.5,2.93,;3.51,1.39,;4.27,2.73,;5.07,1.86,;6.17,2.95,;6.18,1.41,;4.85,.63,;4.85,-.9,;6.19,-1.67,;6.2,-3.21,;4.86,-3.98,;3.53,-3.22,;2.2,-4,;3.52,-1.68,;4.87,-5.52,;3.54,-6.29,;3.54,-7.84,;4.87,-8.61,;6.21,-7.84,;7.68,-8.31,;8.59,-7.06,;7.68,-5.8,;6.21,-6.28,;10.13,-7.06,;10.9,-8.39,;12.44,-8.39,;13.22,-7.06,;12.44,-5.72,;10.9,-5.72,;8.16,-9.78,;9.66,-10.09,;10.14,-11.55,;9.11,-12.71,;7.6,-12.39,;6.35,-13.29,;5.12,-12.38,;5.6,-10.92,;7.13,-10.93,)| Show InChI InChI=1S/C31H27FN8/c1-38-17-22-6-5-21(38)18-39(22)20-7-8-24(26(32)15-20)28-11-14-34-31-29(23-3-2-4-27-25(23)16-35-36-27)30(37-40(28)31)19-9-12-33-13-10-19/h2-4,7-16,21-22H,5-6,17-18H2,1H3,(H,35,36)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Bos taurus) | BDBM50170292
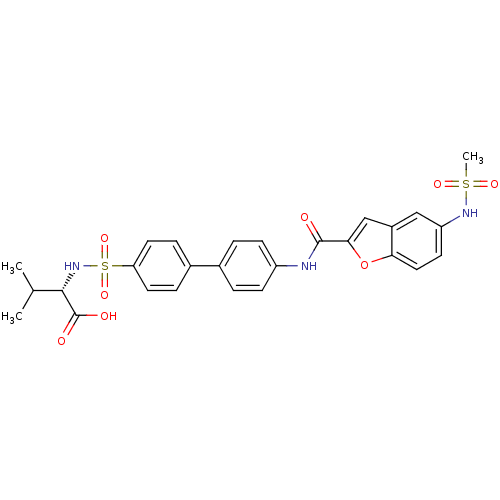
((S)-2-{4'-[(5-Methanesulfonylamino-benzofuran-2-ca...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3cc(NS(C)(=O)=O)ccc3o2)cc1)C(O)=O Show InChI InChI=1S/C27H27N3O8S2/c1-16(2)25(27(32)33)30-40(36,37)22-11-6-18(7-12-22)17-4-8-20(9-5-17)28-26(31)24-15-19-14-21(29-39(3,34)35)10-13-23(19)38-24/h4-16,25,29-30H,1-3H3,(H,28,31)(H,32,33)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 of bovine articular cartilage explants |
Bioorg Med Chem Lett 15: 4105-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.019
BindingDB Entry DOI: 10.7270/Q2TX3DXK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50311989
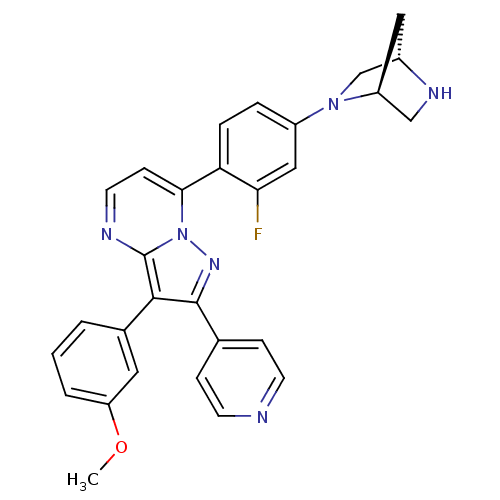
(7-(4-((1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-yl)-...)Show SMILES COc1cccc(c1)-c1c(nn2c(ccnc12)-c1ccc(cc1F)N1C[C@@H]2C[C@H]1CN2)-c1ccncc1 |r| Show InChI InChI=1S/C29H25FN6O/c1-37-23-4-2-3-19(13-23)27-28(18-7-10-31-11-8-18)34-36-26(9-12-32-29(27)36)24-6-5-21(15-25(24)30)35-17-20-14-22(35)16-33-20/h2-13,15,20,22,33H,14,16-17H2,1H3/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50311984
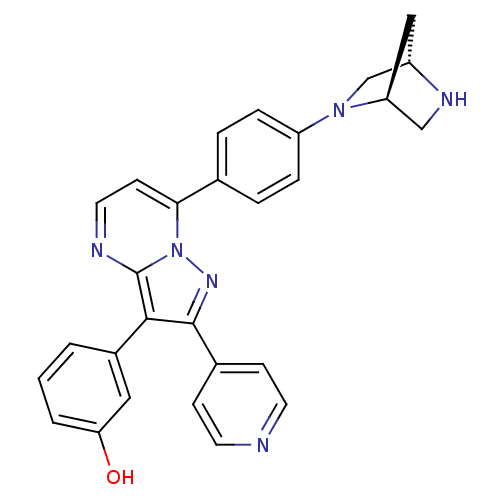
(3-(7-(4-((1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-y...)Show SMILES Oc1cccc(c1)-c1c(nn2c(ccnc12)-c1ccc(cc1)N1C[C@@H]2C[C@H]1CN2)-c1ccncc1 |r| Show InChI InChI=1S/C28H24N6O/c35-24-3-1-2-20(14-24)26-27(19-8-11-29-12-9-19)32-34-25(10-13-30-28(26)34)18-4-6-22(7-5-18)33-17-21-15-23(33)16-31-21/h1-14,21,23,31,35H,15-17H2/t21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
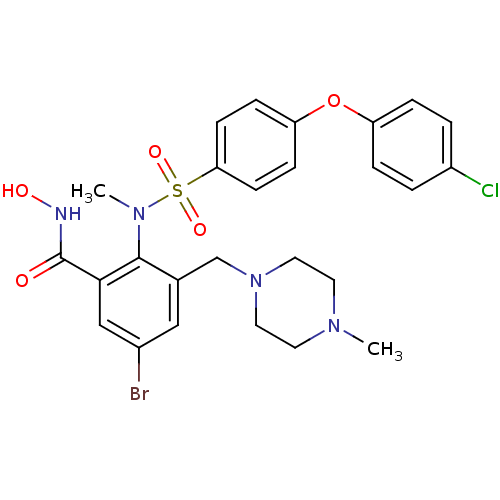
(Homo sapiens (Human)) | BDBM50106133
(5-Bromo-2-{[4-(4-chloro-phenoxy)-benzenesulfonyl]-...)Show SMILES CN(c1c(CN2CCN(C)CC2)cc(Br)cc1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H28BrClN4O5S/c1-30-11-13-32(14-12-30)17-18-15-19(27)16-24(26(33)29-34)25(18)31(2)38(35,36)23-9-7-22(8-10-23)37-21-5-3-20(28)4-6-21/h3-10,15-16,34H,11-14,17H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
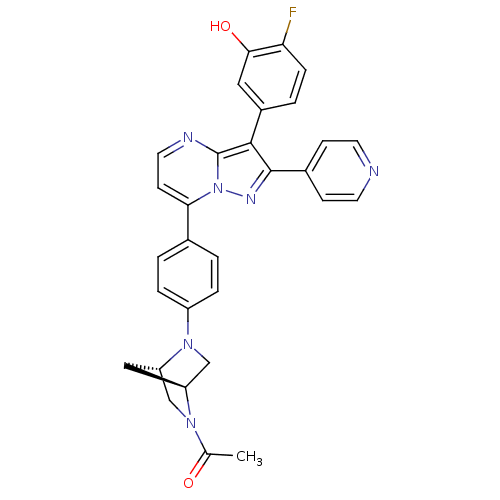
(Homo sapiens (Human)) | BDBM50311987
(1-((1S,4S)-5-(4-(3-(4-fluoro-3-hydroxyphenyl)-2-(p...)Show SMILES CC(=O)N1C[C@@H]2C[C@H]1CN2c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(F)c(O)c1 |r| Show InChI InChI=1S/C30H25FN6O2/c1-18(38)35-16-24-15-23(35)17-36(24)22-5-2-19(3-6-22)26-10-13-33-30-28(21-4-7-25(31)27(39)14-21)29(34-37(26)30)20-8-11-32-12-9-20/h2-14,23-24,39H,15-17H2,1H3/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303100

(2-chloro-5-(7-(8-ethyl-8-azabicyclo[3.2.1]octan-3-...)Show SMILES CCN1C2CCC1CC(C2)c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(Cl)c(O)c1 |THB:10:8:2:4.5| Show InChI InChI=1S/C26H26ClN5O/c1-2-31-19-4-5-20(31)14-18(13-19)22-9-12-29-26-24(17-3-6-21(27)23(33)15-17)25(30-32(22)26)16-7-10-28-11-8-16/h3,6-12,15,18-20,33H,2,4-5,13-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330920
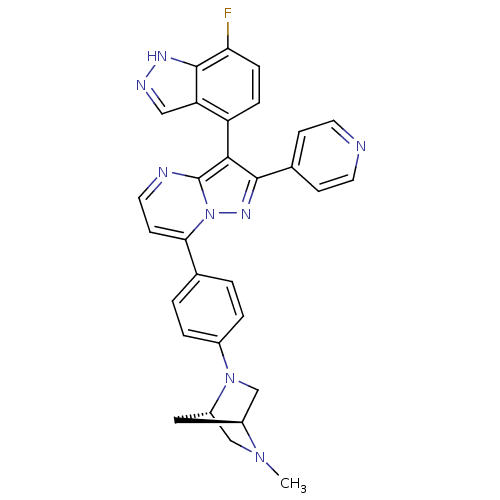
(3-(7-fluoro-1H-indazol-4-yl)-7-(4-((1S,4S)-5-methy...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(F)c2[nH]ncc12 |r,wU:3.3,5.4,(28.5,5.92,;28.51,4.38,;27.17,3.6,;27.18,2.06,;28.27,3.16,;29.85,3.62,;29.85,2.08,;28.52,1.3,;28.53,-.23,;29.86,-1,;29.87,-2.54,;28.53,-3.31,;27.2,-2.55,;27.19,-1.01,;28.54,-4.85,;27.21,-5.62,;27.21,-7.17,;28.55,-7.94,;29.88,-7.16,;31.36,-7.64,;32.27,-6.39,;31.35,-5.13,;29.88,-5.61,;33.8,-6.38,;34.57,-7.72,;36.11,-7.72,;36.89,-6.39,;36.11,-5.05,;34.57,-5.05,;31.83,-9.11,;33.33,-9.42,;33.81,-10.88,;32.78,-12.04,;33.26,-13.5,;31.27,-11.72,;30.03,-12.61,;28.79,-11.71,;29.27,-10.25,;30.8,-10.25,)| Show InChI InChI=1S/C30H25FN8/c1-37-16-22-14-21(37)17-38(22)20-4-2-18(3-5-20)26-10-13-33-30-27(23-6-7-25(31)29-24(23)15-34-35-29)28(36-39(26)30)19-8-11-32-12-9-19/h2-13,15,21-22H,14,16-17H2,1H3,(H,34,35)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50311986
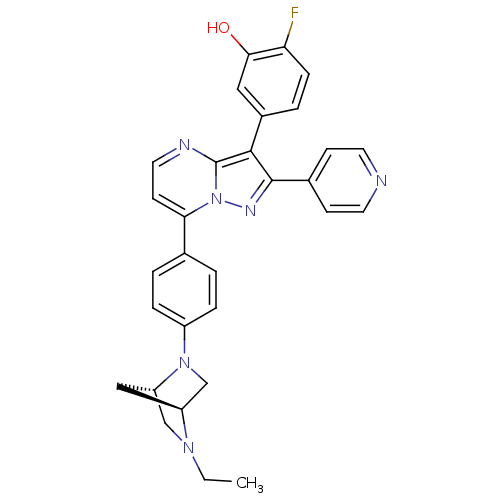
(5-(7-(4-((1S,4S)-5-ethyl-2,5-diazabicyclo[2.2.1]he...)Show SMILES CCN1C[C@@H]2C[C@H]1CN2c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(F)c(O)c1 |r| Show InChI InChI=1S/C30H27FN6O/c1-2-35-17-24-16-23(35)18-36(24)22-6-3-19(4-7-22)26-11-14-33-30-28(21-5-8-25(31)27(38)15-21)29(34-37(26)30)20-9-12-32-13-10-20/h3-15,23-24,38H,2,16-18H2,1H3/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant B-Raf expressed in Sf9 cells assessed as inhibition of Mek1 phosphorylation |
Bioorg Med Chem Lett 19: 6571-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.030
BindingDB Entry DOI: 10.7270/Q29Z9510 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330919
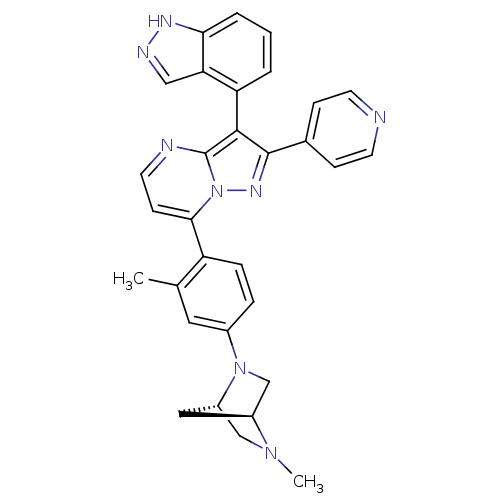
(3-(1H-indazol-4-yl)-7-(2-methyl-4-((1S,4S)-5-methy...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(c(C)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(14.11,6.43,;14.12,4.89,;12.79,4.1,;12.8,2.57,;13.88,3.67,;15.46,4.12,;15.47,2.58,;14.13,1.81,;14.14,.27,;15.48,-.49,;15.48,-2.03,;14.15,-2.81,;12.81,-2.05,;11.48,-2.82,;12.8,-.51,;14.16,-4.34,;12.83,-5.11,;12.83,-6.66,;14.16,-7.43,;15.49,-6.66,;16.97,-7.14,;17.88,-5.88,;16.97,-4.63,;15.49,-5.11,;19.42,-5.88,;20.19,-7.22,;21.73,-7.22,;22.5,-5.88,;21.72,-4.54,;20.18,-4.55,;17.45,-8.6,;18.95,-8.92,;19.43,-10.38,;18.4,-11.53,;16.89,-11.21,;15.64,-12.11,;14.4,-11.2,;14.88,-9.74,;16.42,-9.75,)| Show InChI InChI=1S/C31H28N8/c1-19-14-21(38-18-22-15-23(38)17-37(22)2)6-7-24(19)28-10-13-33-31-29(25-4-3-5-27-26(25)16-34-35-27)30(36-39(28)31)20-8-11-32-12-9-20/h3-14,16,22-23H,15,17-18H2,1-2H3,(H,34,35)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330918
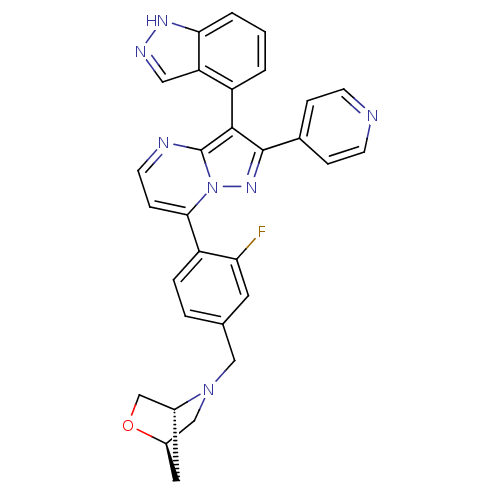
((1R,4R)-5-(4-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)...)Show SMILES Fc1cc(CN2C[C@H]3C[C@@H]2CO3)ccc1-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wD:9.8,7.7,(-13.19,-23.21,;-11.86,-22.44,;-11.88,-20.9,;-10.53,-20.12,;-10.54,-18.57,;-9.21,-17.8,;-7.88,-18.58,;-6.54,-17.83,;-7.7,-16.67,;-9.19,-16.26,;-7.86,-15.5,;-6.52,-16.28,;-9.19,-20.88,;-9.19,-22.42,;-10.52,-23.2,;-10.51,-24.73,;-11.85,-25.5,;-11.85,-27.05,;-10.51,-27.82,;-9.18,-27.05,;-7.7,-27.53,;-6.79,-26.27,;-7.7,-25.02,;-9.18,-25.5,;-5.25,-26.27,;-4.48,-27.61,;-2.94,-27.61,;-2.17,-26.27,;-2.95,-24.93,;-4.49,-24.94,;-7.23,-28.99,;-5.72,-29.31,;-5.24,-30.77,;-6.27,-31.92,;-7.78,-31.6,;-9.03,-32.5,;-10.27,-31.59,;-9.79,-30.13,;-8.25,-30.14,)| Show InChI InChI=1S/C30H24FN7O/c31-25-12-18(15-37-16-21-13-20(37)17-39-21)4-5-23(25)27-8-11-33-30-28(22-2-1-3-26-24(22)14-34-35-26)29(36-38(27)30)19-6-9-32-10-7-19/h1-12,14,20-21H,13,15-17H2,(H,34,35)/t20-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM26526
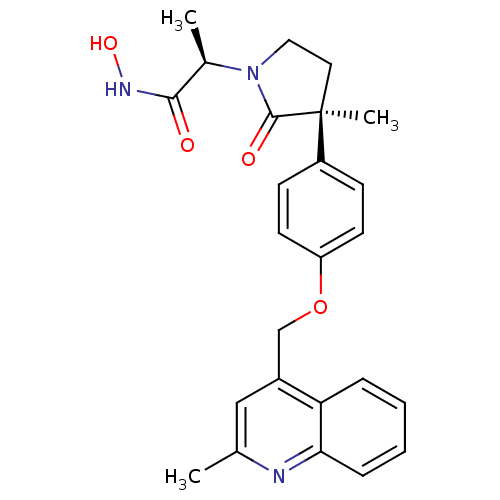
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of TACE in human whole blood assay |
Bioorg Med Chem Lett 17: 34-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.10.004
BindingDB Entry DOI: 10.7270/Q2862G2M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118983

(1-Benzoyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-te...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccccc1 Show InChI InChI=1S/C24H23N3O6S/c1-33-19-11-13-20(14-12-19)34(31,32)27-15-18-9-5-6-10-21(18)26(16-22(27)23(28)25-30)24(29)17-7-3-2-4-8-17/h2-14,22,30H,15-16H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50303131
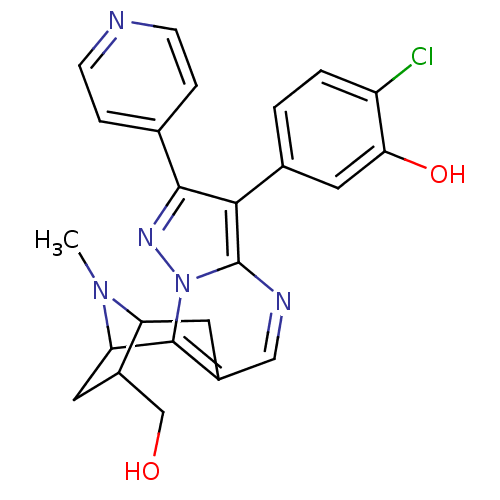
(2-chloro-5-[13-(hydroxymethyl)-15-methyl-5-(pyridi...)Show SMILES CN1C2Cc3cnc4c(c(nn4c3C1CC2CO)-c1ccncc1)-c1ccc(Cl)c(O)c1 |TLB:11:12:1:15.14,THB:5:4:1:15.14| Show InChI InChI=1S/C24H22ClN5O2/c1-29-18-8-15-11-27-24-21(14-2-3-17(25)20(32)10-14)22(13-4-6-26-7-5-13)28-30(24)23(15)19(29)9-16(18)12-31/h2-7,10-11,16,18-19,31-32H,8-9,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 19: 6957-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.058
BindingDB Entry DOI: 10.7270/Q2833S34 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data